Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
- Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2024;39(2):353-363. Published online January 23, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1809

- 904 View
- 40 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Polyunsaturated fatty acids (PUFAs) reportedly have protective effects on pancreatic β-cells; however, the underlying mechanisms are unknown.
Methods
To investigate the cellular mechanism of PUFA-induced cell protection, mouse insulinoma 6 (MIN6) cells were cultured with palmitic acid (PA) and/or docosahexaenoic acid (DHA), and alterations in cellular signaling and apoptosis were examined.
Results
DHA treatment remarkably repressed caspase-3 cleavage and terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL)-positive red dot signals in PA-treated MIN6 cells, with upregulation of autophagy, an increase in microtubule- associated protein 1-light chain 3 (LC3)-II, autophagy-related 5 (Atg5), and decreased p62. Upstream factors involved in autophagy regulation (Beclin-1, unc51 like autophagy activating kinase 1 [ULK1], phosphorylated mammalian target of rapamycin [mTOR], and protein kinase B) were also altered by DHA treatment. DHA specifically induced phosphorylation on S2448 in mTOR; however, phosphorylation on S2481 decreased. The role of G protein-coupled receptor 120 (GPR120) in the effect of DHA was demonstrated using a GPR120 agonist and antagonist. Additional treatment with AH7614, a GPR120 antagonist, significantly attenuated DHA-induced autophagy and protection. Taken together, DHA-induced autophagy activation with protection against PA-induced apoptosis mediated by the GPR120/mTOR axis.
Conclusion
These findings indicate that DHA has therapeutic effects on PA-induced pancreatic β-cells, and that the cellular mechanism of β-cell protection by DHA may be a new research target with potential pharmacotherapeutic implications in β-cell protection.

- Diabetes, Obesity and Metabolism
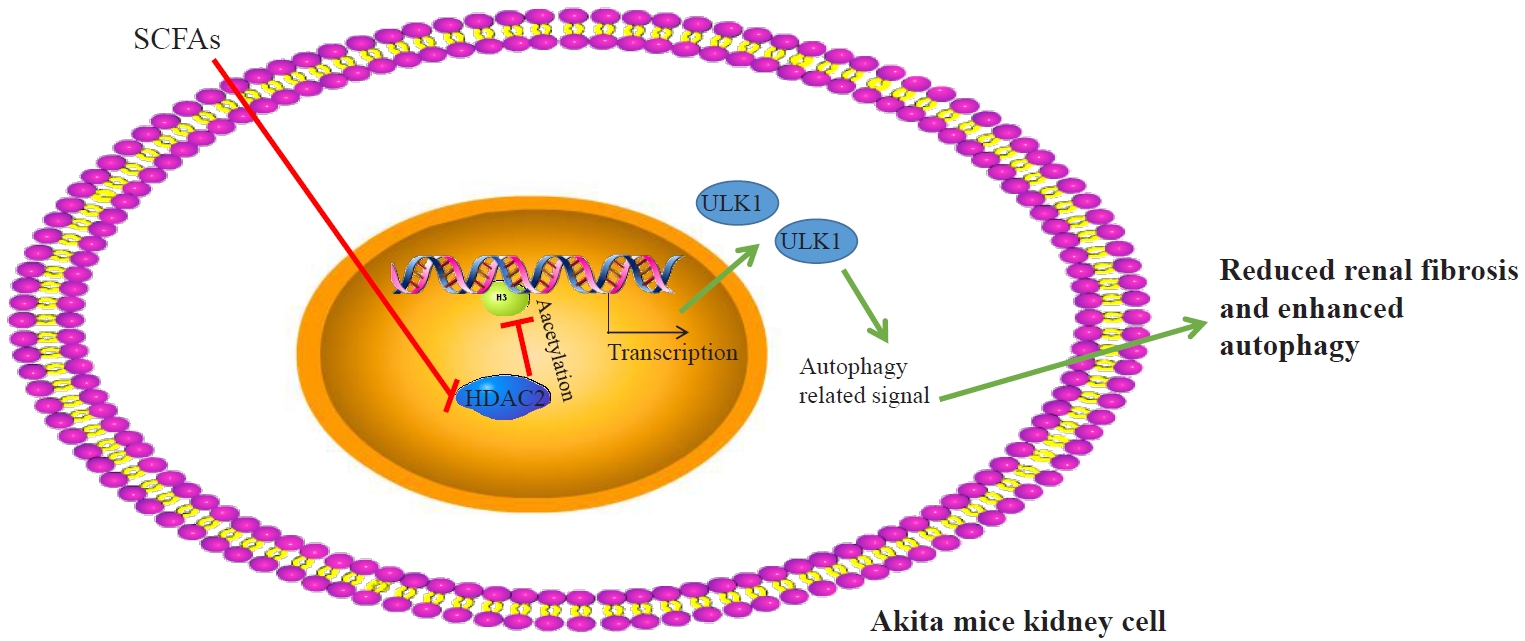
- Short-Chain Fatty Acids Attenuate Renal Fibrosis and Enhance Autophagy of Renal Tubular Cells in Diabetic Mice Through the HDAC2/ULK1 Axis
- Xiaoying Ma, Qiong Wang
- Endocrinol Metab. 2022;37(3):432-443. Published online May 16, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1336
- 7,052 View
- 152 Download
- 10 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study investigated the effect of short-chain fatty acids (SCFAs) on diabetes in a mouse model.
Methods
Autophagy in Akita mice and streptozocin (STZ)-induced diabetic C57BL/6 mice was determined by Western blots and immunohistochemistry (IHC). Western blots, IHC, hematoxylin and eosin staining, Masson staining, periodic acid-Schiff staining, and picrosirius red staining were conducted to detect whether autophagy and renal function improved in Akita mice and STZ-induced diabetic C57BL/6 mice after treatment of SCFAs. Western blots, IHC, and chromatin immunoprecipitation were performed to determine whether SCFAs affected diabetic mice via the histone deacetylase (HDAC2)/unc-51 like autophagy activating kinase 1 (ULK1) axis. Diabetic mice with kidney-specific knockout of HDAC2 were constructed, and IHC, Masson staining, and Western blots were carried out to detect whether the deletion of endogenous HDAC2 contributed to the improvement of autophagy and renal fibrosis in diabetic mice.
Results
Reduced autophagy and severe fibrosis were observed in Akita mice and STZ-induced diabetic C57BL/6 mice. Increased autophagy and reduced renal cell fibrosis were found in SCFA-treated Akita diabetic mice and STZ-induced diabetic C57BL/6 mice. Diabetic mice treated with SCFAs had lower HDAC2 expression and more enriched binding of ULK1 promoter sequences to H3K27Ac. Endogenous knockout of HDAC2 caused enhanced autophagy and decreased renal fibrosis in diabetic mice treated with SCFAs.
Conclusion
SCFAs enhanced autophagy of renal tubular cells and attenuated renal fibrosis in diabetic mice through the HDAC2/ULK1 axis. -
Citations
Citations to this article as recorded by- NSD1 supports cell growth and regulates autophagy in HPV-negative head and neck squamous cell carcinoma
Iuliia Topchu, Igor Bychkov, Demirkan Gursel, Petr Makhov, Yanis Boumber
Cell Death Discovery.2024;[Epub] CrossRef - Dietary fiber intake and its association with diabetic kidney disease in American adults with diabetes: A cross-sectional study
Xin-Hua Jia, Sheng-Yan Wang, Ai-Qin Sun
World Journal of Diabetes.2024; 15(3): 475. CrossRef - Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets
Feng Shu, Han Xiao, Qiu-Nuo Li, Xiao-Shuai Ren, Zhi-Gang Liu, Bo-Wen Hu, Hong-Sheng Wang, Hao Wang, Guan-Min Jiang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids
Ekaterina Fock, Rimma Parnova
Cells.2023; 12(4): 657. CrossRef - The Role of Histone Modifications in the Pathogenesis of Diabetic Kidney Disease
Christodoula Kourtidou, Konstantinos Tziomalos
International Journal of Molecular Sciences.2023; 24(6): 6007. CrossRef - Mechanism of histone deacetylase HDAC2 in FOXO3-mediated trophoblast pyroptosis in preeclampsia
Jia Liu, Weihui Yang
Functional & Integrative Genomics.2023;[Epub] CrossRef - Macrophage polarization induces endothelium-to-myofibroblast transition in chronic allograft dysfunction
Zeping Gui, Xiang Zhang, Qianguang Han, Zhou Hang, Ruoyun Tan, Min Gu, Zijie Wang
Renal Failure.2023;[Epub] CrossRef - Periodic acid–Schiff staining in oral exfoliative cytology of diabetic patients: The odyssey for noninvasive screening – A systematic review and meta-analysis
KYesoda Aniyan, KrithikaChandrasekar Lakshmi, Anuradha Ganesan
Dental Research Journal.2023; 20(1): 73. CrossRef - Luteolin alleviates renal ischemia-reperfusion injury in streptozotocin induced diabetic rats by inhibiting metalloenzymes expression
Rakesh B. Daude, Jigna S. Shah
Current Issues in Pharmacy and Medical Sciences.2023; 36(4): 199. CrossRef - Molecular mechanisms of histone deacetylases and inhibitors in renal fibrosis progression
Jiayu Wang, Jiaxing Li, Xin Zhang, Min Zhang, Xiaopeng Hu, Hang Yin
Frontiers in Molecular Biosciences.2022;[Epub] CrossRef - Sacubitril/Valsartan contributes to improving the diabetic kidney disease and regulating the gut microbiota in mice
Peipei Wang, Ruixue Guo, Xiwen Bai, Wen Cui, Yiding Zhang, Huangmin Li, Jin Shang, Zhanzheng Zhao
Frontiers in Endocrinology.2022;[Epub] CrossRef - Recent advances and potentiality of postbiotics in the food industry: Composition, inactivation methods, current applications in metabolic syndrome, and future trends
Yujie Zhong, Tao Wang, Ruilin Luo, Jiayu Liu, Ruyi Jin, Xiaoli Peng
Critical Reviews in Food Science and Nutrition.2022; : 1. CrossRef
- NSD1 supports cell growth and regulates autophagy in HPV-negative head and neck squamous cell carcinoma

- Endocrine Research
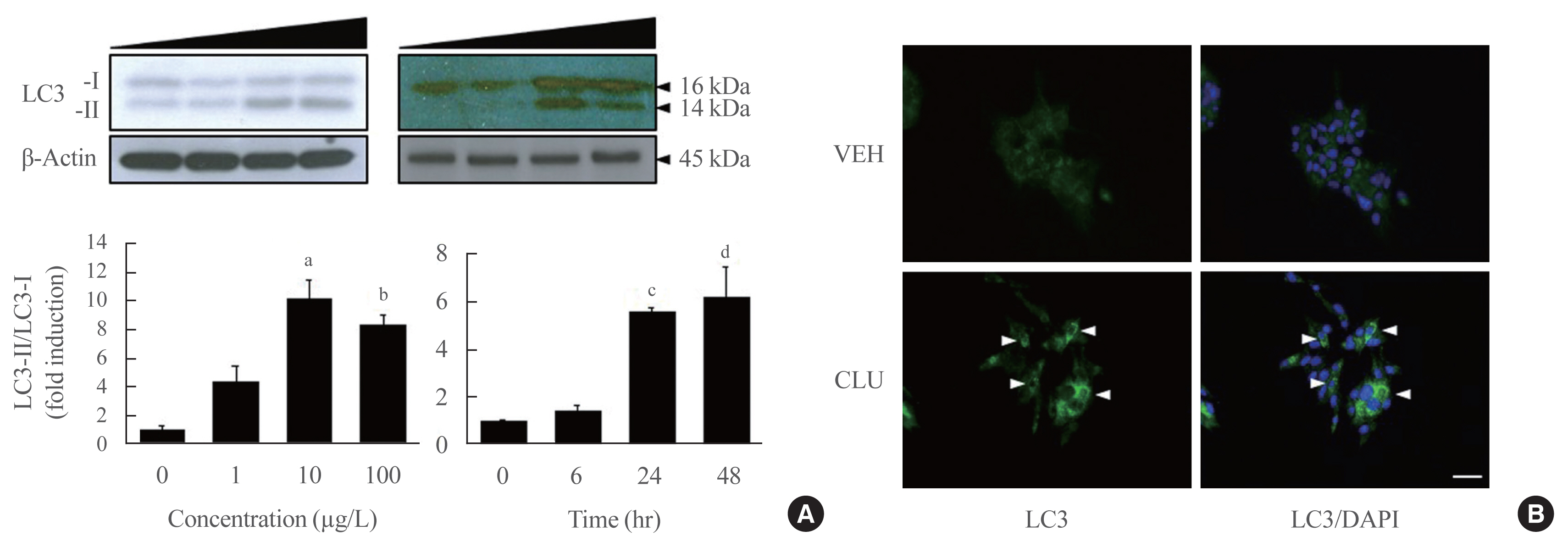
- Clusterin Protects Lipotoxicity-Induced Apoptosis via Upregulation of Autophagy in Insulin-Secreting Cells
- Seok-Woo Hong, Jinmi Lee, Min Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2020;35(4):943-953. Published online December 2, 2020
- DOI: https://doi.org/10.3803/EnM.2020.768
- 5,665 View
- 135 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is a great need to discover factors that could protect pancreatic β-cells from apoptosis and thus prevent diabetes mellitus. Clusterin (CLU), a chaperone protein, plays an important role in cell protection in numerous cells and is involved in various cellular mechanisms, including autophagy. In the present study, we investigated the protective role of CLU through autophagy regulation in pancreatic β-cells.
Methods
To identify the protective role of CLU, mouse insulinoma 6 (MIN6) cells were incubated with CLU and/or free fatty acid (FFA) palmitate, and cellular apoptosis and autophagy were examined.
Results
Treatment with CLU remarkably upregulated microtubule-associated protein 1-light chain 3 (LC3)-II conversion in a doseand time-dependent manner with a significant increase in the autophagy-related 3 (Atg3) gene expression level, which is a mediator of LC3-II conversion. Moreover, co-immunoprecipitation and fluorescence microscopy experiments showed that the molecular interaction of LC3 with Atg3 and p62 was markedly increased by CLU. Stimulation of LC3-II conversion by CLU persisted in lipotoxic conditions, and FFA-induced apoptosis and dysfunction were simultaneously improved by CLU treatment. Finally, inhibition of LC3-II conversion by Atg3 gene knockdown markedly attenuated the cytoprotective effect of CLU.
Conclusion
Taken together, these findings suggest that CLU protects pancreatic β-cells against lipotoxicity-induced apoptosis via autophagy stimulation mediated by facilitating LC3-II conversion. Thus, CLU has therapeutic effects on FFA-induced pancreatic β-cell dysfunction. -
Citations
Citations to this article as recorded by- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes
Alexandra Coomans de Brachène, Corentin Scoubeau, Anyïshai E. Musuaya, Jose Maria Costa-Junior, Angela Castela, Julie Carpentier, Vitalie Faoro, Malgorzata Klass, Miriam Cnop, Decio L. Eizirik
Diabetologia.2023; 66(3): 450. CrossRef - Apolipoprotein J Attenuates Vascular Restenosis by Promoting Autophagy and Inhibiting the Proliferation and Migration of Vascular Smooth Muscle Cells
Ning Yang, Bo Dong, Yanqiu Song, Yang Li, Lu Kou, Qin Qin
Journal of Cardiovascular Translational Research.2022; 15(5): 1086. CrossRef - Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
Seok-Woo Hong, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 57. CrossRef - Co-regulators of autophagy and the cell cycle in HFD − As treated mice
Marzieh Zeinvand-Lorestani, Mohammad Javad Khodayar, Ali Teimoori, Najmaldin Saki, Akram Ahangarpour, Ali Ranjbar, Hamed Zeinvand-Lorestani
Journal of Trace Elements and Minerals.2022; 2: 100018. CrossRef - Targeting pancreatic β cells for diabetes treatment
Chirag Jain, Ansarullah, Sara Bilekova, Heiko Lickert
Nature Metabolism.2022; 4(9): 1097. CrossRef - Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies
Safia Costes, Gyslaine Bertrand, Magalie A. Ravier
International Journal of Molecular Sciences.2021; 22(10): 5303. CrossRef
- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes

- Obesity and Metabolism
- Mitochondrial Complexes I and II Are More Susceptible to Autophagy Deficiency in Mouse β-Cells
- Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Min Kyeong Kim, Jung Hee Kim, Masaaki Komatsu, Keiji Tanaka, Hakmo Lee, Sung Soo Chung, Soo Heon Kwak, Young Min Cho, Kyong Soo Park, Hye Seung Jung
- Endocrinol Metab. 2015;30(1):65-70. Published online March 27, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.1.65
- 3,959 View
- 40 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Damaged mitochondria are removed by autophagy. Therefore, impairment of autophagy induces the accumulation of damaged mitochondria and mitochondrial dysfunction in most mammalian cells. Here, we investigated mitochondrial function and the expression of mitochondrial complexes in autophagy-related 7 (
Atg7 )-deficient β-cells.Methods To evaluate the effect of autophagy deficiency on mitochondrial function in pancreatic β-cells, we isolated islets from
Atg7 F/F:RIP-Cre + mice and wild-type littermates. Oxygen consumption rate and intracellular adenosine 5'-triphosphate (ATP) content were measured. The expression of mitochondrial complex genes inAtg7 -deficient islets and in β-TC6 cells transfected with siAtg7 was measured by quantitative real-time polymerase chain reaction.Results Baseline oxygen consumption rate of
Atg7 -deficient islets was significantly lower than that of control islets (P <0.05). Intracellular ATP content ofAtg7 -deficient islets during glucose stimulation was also significantly lower than that of control islets (P <0.05). By Oxygraph-2k analysis, mitochondrial respiration inAtg7 -deficient islets was significantly decreased overall, although state 3 respiration and responses to antimycin A were unaffected. The mRNA levels of mitochondrial complexes I, II, III, and V inAtg7 -deficient islets were significantly lower than in control islets (P <0.05). Down-regulation ofAtg7 in β-TC6 cells also reduced the expression of complexes I and II, with marginal significance (P <0.1).Conclusion Impairment of autophagy in pancreatic β-cells suppressed the expression of some mitochondrial respiratory complexes, and may contribute to mitochondrial dysfunction. Among the complexes, I and II seem to be most vulnerable to autophagy deficiency.
-
Citations
Citations to this article as recorded by- Proteomic pathways to metabolic disease and type 2 diabetes in the pancreatic islet
Belinda Yau, Sheyda Naghiloo, Alexis Diaz-Vegas, Austin V. Carr, Julian Van Gerwen, Elise J. Needham, Dillon Jevon, Sing-Young Chen, Kyle L. Hoehn, Amanda E. Brandon, Laurence Macia, Gregory J. Cooney, Michael R. Shortreed, Lloyd M. Smith, Mark P. Keller,
iScience.2021; 24(10): 103099. CrossRef - Natural compound oblongifolin C inhibits autophagic flux, and induces apoptosis and mitochondrial dysfunction in human cholangiocarcinoma QBC939 cells
Aiqing Zhang, Wei He, Huimin Shi, Xiaodan Huang, Guozhong Ji
Molecular Medicine Reports.2016; 14(4): 3179. CrossRef - Autophagy deficiency in β cells blunts incretin-induced suppression of glucagon release from α cells
Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Hakmo Lee, Sung Soo Chung, Dong-Sik Ham, Ji-Won Kim, Kun-Ho Yoon, Kyong Soo Park, Hye Seung Jung
Islets.2015; 7(5): e1129096. CrossRef
- Proteomic pathways to metabolic disease and type 2 diabetes in the pancreatic islet

- Obesity and Metabolism
- Role of Autophagy in the Control of Body Metabolism
- Wenying Quan, Myung-Shik Lee
- Endocrinol Metab. 2013;28(1):6-11. Published online March 25, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.1.6
- 3,929 View
- 51 Download
- 26 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Autophagy plays a crucial role in the maintenance of cellular nutrient balance and the function of organelles such as mitochondria or the endoplasmic reticulum, which are important in intracellular metabolism, insulin release, and insulin sensitivity. In the insulin-producing pancreatic β-cells, autophagy is important in the maintenance of β-cell mass, structure, and function. Mice with deficiencies in β-cell-specific autophagy show reduced β-cell mass and defects in insulin secretion that lead to hypoinsulinemia and hyperglycemia but not diabetes. However, these mice developed diabetes when bred with
ob/ob mice, suggesting that autophagy-deficient β-cells have defects in dealing with the increased metabolic stress imposed by obesity. These results also imply that autophagy deficiency in β-cells could be a factor in the progression from obesity to diabetes. Another important function of autophagy is in hypothalamic neurons for the central control of energy expenditure, appetite, and body weight. In addition, mice with autophagy deficiencies in the target tissues of insulin have yielded diverse phenotypes. Taken together, these results suggest that autophagy is important in the control of whole body energy and nutrient homeostasis, and its dysregulation could play a role in the development of metabolic disorders and diabetes.-
Citations
Citations to this article as recorded by- Anti‐influenza A (H1N1) virus effect of gallic acid through inhibition of virulent protein production and association with autophagy
Cheng‐Chieh Chang, Huey‐Ling You, Huey‐Jen Su, I‐Ling Hung, Chao‐Wei Kao, Sheng‐Teng Huang
Food Science & Nutrition.2024; 12(3): 1605. CrossRef - The Effect of Synthetic Curcumin Analogues on Obesity, Diabetes
and Cardiovascular Disease: A Literature Review
Salime Lavian, Pegah Mardaneh, Mohammad Bagherniya, Seyed Ahmad Emami, Alexandra E. Butler, Amirhossein Sahebkar
Current Medicinal Chemistry.2023; 30(35): 3979. CrossRef - PERK prevents hepatic lipotoxicity by activating the p62-ULK1 axis-mediated noncanonical KEAP1-Nrf2 pathway
Da Hyun Lee, Jeong Su Park, Yu Seol Lee, Soo Han Bae
Redox Biology.2022; 50: 102235. CrossRef - Autophagy in the Neuronal Ceroid Lipofuscinoses (Batten Disease)
William D. Kim, Morgan L. D. M. Wilson-Smillie, Aruban Thanabalasingam, Stephane Lefrancois, Susan L. Cotman, Robert J. Huber
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Investigation and experimental validation of curcumin-related mechanisms against hepatocellular carcinoma based on network pharmacology
Yang Chen, Qian Li, Sisi Ren, Ting Chen, Bingtao Zhai, Jiangxue Cheng, Xiaoyan Shi, Liang Song, Yu Fan, Dongyan Guo
Journal of Zhejiang University-SCIENCE B.2022; 23(8): 682. CrossRef - Computational prediction and experimental validation of Salmonella Typhimurium SopE-mediated fine-tuning of autophagy in intestinal epithelial cells
Amanda Demeter, Anne-Claire Jacomin, Lejla Gul, Ashleigh Lister, James Lipscombe, Rachele Invernizzi, Priscilla Branchu, Iain Macaulay, Ioannis P. Nezis, Robert A. Kingsley, Tamas Korcsmaros, Isabelle Hautefort
Frontiers in Cellular and Infection Microbiology.2022;[Epub] CrossRef - At the heart of mitochondrial quality control: many roads to the top
Roberta A. Gottlieb, Honit Piplani, Jon Sin, Savannah Sawaged, Syed M. Hamid, David J. Taylor, Juliana de Freitas Germano
Cellular and Molecular Life Sciences.2021; 78(8): 3791. CrossRef - Spontaneous preterm birth: the underpinnings in the maternal and fetal genomes
Esha Bhattacharjee, Arindam Maitra
npj Genomic Medicine.2021;[Epub] CrossRef - Regular football training down-regulates miR-1303 muscle expression in veterans
A. Mancini, D. Vitucci, F. M. Orlandella, A. Terracciano, R. M. Mariniello, E. Imperlini, E. Grazioli, S. Orrù, P. Krustrup, G. Salvatore, P. Buono
European Journal of Applied Physiology.2021; 121(10): 2903. CrossRef - Catechin inhibiting the H1N1 influenza virus associated with the regulation of autophagy
Cheng-Chieh Chang, Huey-Ling You, Sheng-Teng Huang
Journal of the Chinese Medical Association.2020; 83(4): 386. CrossRef - Silencing of PARP2 Blocks Autophagic Degradation
Laura Jankó, Zsanett Sári, Tünde Kovács, Gréta Kis, Magdolna Szántó, Miklós Antal, Gábor Juhász, Péter Bai
Cells.2020; 9(2): 380. CrossRef - Adipose-specific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release
Fang Li, Huixia Li, Xinxin Jin, Ying Zhang, Xiaomin Kang, Zhuanmin Zhang, Mao Xu, Zhuang Qian, Zhengmin Ma, Xin Gao, Liting Zhao, Shufang Wu, Hongzhi Sun
Cell Cycle.2019; 18(17): 2067. CrossRef - Maternal pentachlorophenol exposure induces developmental toxicity mediated by autophagy on pregnancy mice
Xiaomin Huang, Xiumei Han, Zhenyao Huang, Mingming Yu, Yan Zhang, Yun Fan, Bo Xu, Kun Zhou, Ling Song, Xinru Wang, Chuncheng Lu, Yankai Xia
Ecotoxicology and Environmental Safety.2019; 169: 829. CrossRef - The ciliary protein RPGRIP1L governs autophagy independently of its proteasome-regulating function at the ciliary base in mouse embryonic fibroblasts
Andreas Struchtrup, Antonia Wiegering, Björn Stork, Ulrich Rüther, Christoph Gerhardt
Autophagy.2018; 14(4): 567. CrossRef - Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition
Soo Hyun Kim, Gyuri Kim, Dai Hoon Han, Milim Lee, Irene Kim, Bohkyung Kim, Kook Hwan Kim, Young-Mi Song, Jeong Eun Yoo, Hye Jin Wang, Soo Han Bae, Yong-Ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Myung-Shik Lee
Autophagy.2017; 13(10): 1767. CrossRef - BNIP3 is essential for mitochondrial bioenergetics during adipocyte remodelling in mice
Jin Woo Choi, Anna Jo, Min Kim, Ho Seon Park, Sung Soo Chung, Shinae Kang, Kyong Soo Park
Diabetologia.2016; 59(3): 571. CrossRef - Regulation of autophagy by amino acids and MTOR-dependent signal transduction
Alfred J. Meijer, Séverine Lorin, Edward F. Blommaart, Patrice Codogno
Amino Acids.2015; 47(10): 2037. CrossRef - Antioxidant catalase rescues against high fat diet-induced cardiac dysfunction via an IKKβ-AMPK-dependent regulation of autophagy
Lei Liang, Xi-Ling Shou, Hai-Kang Zhao, Gu-qun Ren, Jian-Bang Wang, Xi-Hui Wang, Wen-Ting Ai, Jackie R. Maris, Lindsay K. Hueckstaedt, Ai-qun Ma, Yingmei Zhang
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(2): 343. CrossRef - Autophagy deficiency in β cells blunts incretin-induced suppression of glucagon release from α cells
Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Hakmo Lee, Sung Soo Chung, Dong-Sik Ham, Ji-Won Kim, Kun-Ho Yoon, Kyong Soo Park, Hye Seung Jung
Islets.2015; 7(5): e1129096. CrossRef - Mitochondrial Complexes I and II Are More Susceptible to Autophagy Deficiency in Mouse β-Cells
Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Min Kyeong Kim, Jung Hee Kim, Masaaki Komatsu, Keiji Tanaka, Hakmo Lee, Sung Soo Chung, Soo Heon Kwak, Young Min Cho, Kyong Soo Park, Hye Seung Jung
Endocrinology and Metabolism.2015; 30(1): 65. CrossRef - Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
Deok-Bae Park
Diabetes & Metabolism Journal.2015; 39(6): 518. CrossRef - Regulation of autophagy by the nuclear factor κB signaling pathway in the hippocampus of rats with sepsis
YunJie Su, Yi Qu, FengYan Zhao, HuaFeng Li, DeZhi Mu, XiHong Li
Journal of Neuroinflammation.2015;[Epub] CrossRef - Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes
Han Liu, Ming-ming Cao, Yang Wang, Le-chen Li, Li-bo Zhu, Guang-ying Xie, Yan-bo Li
General and Comparative Endocrinology.2015; 210: 124. CrossRef - This old heart: Cardiac aging and autophagy
Phyllis-Jean Linton, Michael Gurney, David Sengstock, Robert M. Mentzer, Roberta A. Gottlieb
Journal of Molecular and Cellular Cardiology.2015; 83: 44. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef - Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice
Arnaud Francois, Faraj Terro, Nathalie Quellard, Beatrice Fernandez, Damien Chassaing, Thierry Janet, Agnes Rioux Bilan, Marc Paccalin, Guylene Page
Molecular Brain.2014;[Epub] CrossRef
- Anti‐influenza A (H1N1) virus effect of gallic acid through inhibition of virulent protein production and association with autophagy


 KES
KES

 First
First Prev
Prev



