Search
- Page Path
- HOME > Search
- Endocrine Research
- Clusterin Protects Lipotoxicity-Induced Apoptosis via Upregulation of Autophagy in Insulin-Secreting Cells
- Seok-Woo Hong, Jinmi Lee, Min Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2020;35(4):943-953. Published online December 2, 2020
- DOI: https://doi.org/10.3803/EnM.2020.768
- 5,668 View
- 135 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is a great need to discover factors that could protect pancreatic β-cells from apoptosis and thus prevent diabetes mellitus. Clusterin (CLU), a chaperone protein, plays an important role in cell protection in numerous cells and is involved in various cellular mechanisms, including autophagy. In the present study, we investigated the protective role of CLU through autophagy regulation in pancreatic β-cells.
Methods
To identify the protective role of CLU, mouse insulinoma 6 (MIN6) cells were incubated with CLU and/or free fatty acid (FFA) palmitate, and cellular apoptosis and autophagy were examined.
Results
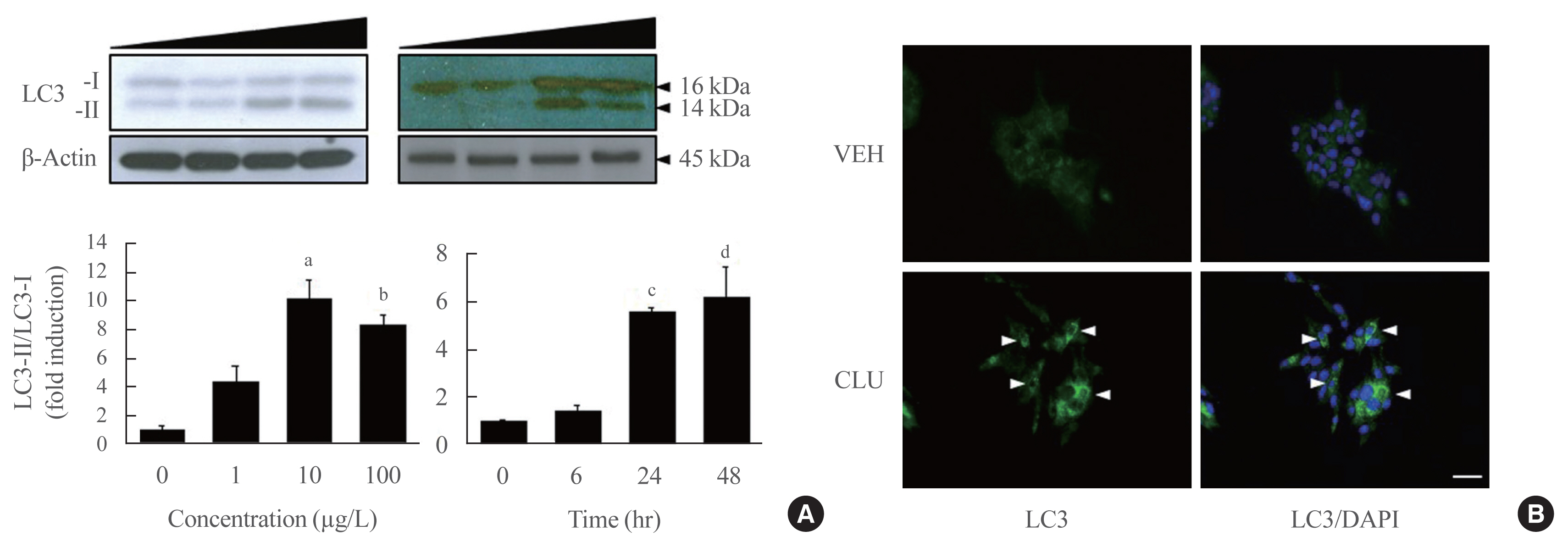
Treatment with CLU remarkably upregulated microtubule-associated protein 1-light chain 3 (LC3)-II conversion in a doseand time-dependent manner with a significant increase in the autophagy-related 3 (Atg3) gene expression level, which is a mediator of LC3-II conversion. Moreover, co-immunoprecipitation and fluorescence microscopy experiments showed that the molecular interaction of LC3 with Atg3 and p62 was markedly increased by CLU. Stimulation of LC3-II conversion by CLU persisted in lipotoxic conditions, and FFA-induced apoptosis and dysfunction were simultaneously improved by CLU treatment. Finally, inhibition of LC3-II conversion by Atg3 gene knockdown markedly attenuated the cytoprotective effect of CLU.
Conclusion
Taken together, these findings suggest that CLU protects pancreatic β-cells against lipotoxicity-induced apoptosis via autophagy stimulation mediated by facilitating LC3-II conversion. Thus, CLU has therapeutic effects on FFA-induced pancreatic β-cell dysfunction. -
Citations
Citations to this article as recorded by- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes
Alexandra Coomans de Brachène, Corentin Scoubeau, Anyïshai E. Musuaya, Jose Maria Costa-Junior, Angela Castela, Julie Carpentier, Vitalie Faoro, Malgorzata Klass, Miriam Cnop, Decio L. Eizirik
Diabetologia.2023; 66(3): 450. CrossRef - Apolipoprotein J Attenuates Vascular Restenosis by Promoting Autophagy and Inhibiting the Proliferation and Migration of Vascular Smooth Muscle Cells
Ning Yang, Bo Dong, Yanqiu Song, Yang Li, Lu Kou, Qin Qin
Journal of Cardiovascular Translational Research.2022; 15(5): 1086. CrossRef - Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
Seok-Woo Hong, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 57. CrossRef - Co-regulators of autophagy and the cell cycle in HFD − As treated mice
Marzieh Zeinvand-Lorestani, Mohammad Javad Khodayar, Ali Teimoori, Najmaldin Saki, Akram Ahangarpour, Ali Ranjbar, Hamed Zeinvand-Lorestani
Journal of Trace Elements and Minerals.2022; 2: 100018. CrossRef - Targeting pancreatic β cells for diabetes treatment
Chirag Jain, Ansarullah, Sara Bilekova, Heiko Lickert
Nature Metabolism.2022; 4(9): 1097. CrossRef - Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies
Safia Costes, Gyslaine Bertrand, Magalie A. Ravier
International Journal of Molecular Sciences.2021; 22(10): 5303. CrossRef
- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes

- Obesity and Metabolism
- Distinct Ultradian Rhythms in Plasma Clusterin Concentrations in Lean and Obese Korean Subjects
- Jong Han Choi, Eunheui Jeong, Byung Soo Youn, Min-Seon Kim
- Endocrinol Metab. 2018;33(2):245-251. Published online May 4, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.245
- 4,508 View
- 42 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Blood levels of many hormones show rhythmic fluctuations with variable duration of cycles. Clusterin/apolipoprotein J is a glycoprotein which is highly expressed in the plasma and has modulatory roles in immune and inflammatory reactions, neurobiology, lipid metabolism, and leptin signaling. In this study, we examined the diurnal fluctuations of plasma clusterin concentrations in lean and obese young men.
Methods For the study, 14 subjects (five lean and five obese men; two lean and two obese women) were admitted to the research ward and blood samples were drawn every 30 minutes during light-on period (6:00 AM to 10:00 PM) and every hour during light-off period.
Results Notably, plasma clusterin concentrations displayed a unique ultradian rhythm with five cycles a day in both men and women. During the light-on period, circulating clusterin levels showed fluctuating curves with 4 hours regular intervals with sharp peaks and troughs. In contrast, single oscillation curve during light-off exhibited a smoothened/lower peak and longer (8-hour) duration. In obese men, these cycles were phase-advanced by approximately 1 hour, and had reduced amplitude of fluctuating curves and blunted diurnal pattern. Cyclic fluctuations of plasma clusterin were preserved under fasting and unexpected meal condition, suggesting that rhythmic oscillations in plasma clusterin levels are not generated by meal-related cues.
Conclusion These findings firstly demonstrate a novel pattern of plasma clusterin fluctuations with extremely regular cycles.
-
Citations
Citations to this article as recorded by- Clusterin and Related Scoring Index as Potential Early Predictors of Response to Sorafenib in Hepatocellular Carcinoma
Satoshi Narahara, Takehisa Watanabe, Katsuya Nagaoka, Nahoko Fujimoto, Yoki Furuta, Kentaro Tanaka, Takayuki Tokunaga, Takeshi Kawasaki, Yoko Yoshimaru, Hiroko Setoyama, Kentaro Oniki, Junji Saruwatari, Masakuni Tateyama, Hideaki Naoe, Motohiko Tanaka, Ya
Hepatology Communications.2022; 6(5): 1198. CrossRef - The role of circadian rhythm in choroid plexus functions
Telma Quintela, André Furtado, Ana C. Duarte, Isabel Gonçalves, Jihwan Myung, Cecília R.A. Santos
Progress in Neurobiology.2021; 205: 102129. CrossRef - A Novel Multi-Biomarker Assay for Non-Invasive Quantitative Monitoring of Kidney Injury
Drew Watson, Joshua Y. C. Yang, Reuben D. Sarwal, Tara K. Sigdel, Juliane M. Liberto, Izabella Damm, Victoria Louie, Shristi Sigdel, Devon Livingstone, Katherine Soh, Arjun Chakraborty, Michael Liang, Pei-Chen Lin, Minnie M. Sarwal
Journal of Clinical Medicine.2019; 8(4): 499. CrossRef
- Clusterin and Related Scoring Index as Potential Early Predictors of Response to Sorafenib in Hepatocellular Carcinoma


 KES
KES

 First
First Prev
Prev



