Search
- Page Path
- HOME > Search
Original Articles
- Diabetes, obesity and metabolism
- Phloretin Ameliorates Succinate-Induced Liver Fibrosis by Regulating Hepatic Stellate Cells
- Cong Thuc Le, Giang Nguyen, So Young Park, Hanh Nguyen Dong, Yun Kyung Cho, Jae-Ho Lee, Seung-Soon Im, Dae-Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2023;38(4):395-405. Published online August 3, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1661
- 1,513 View
- 105 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
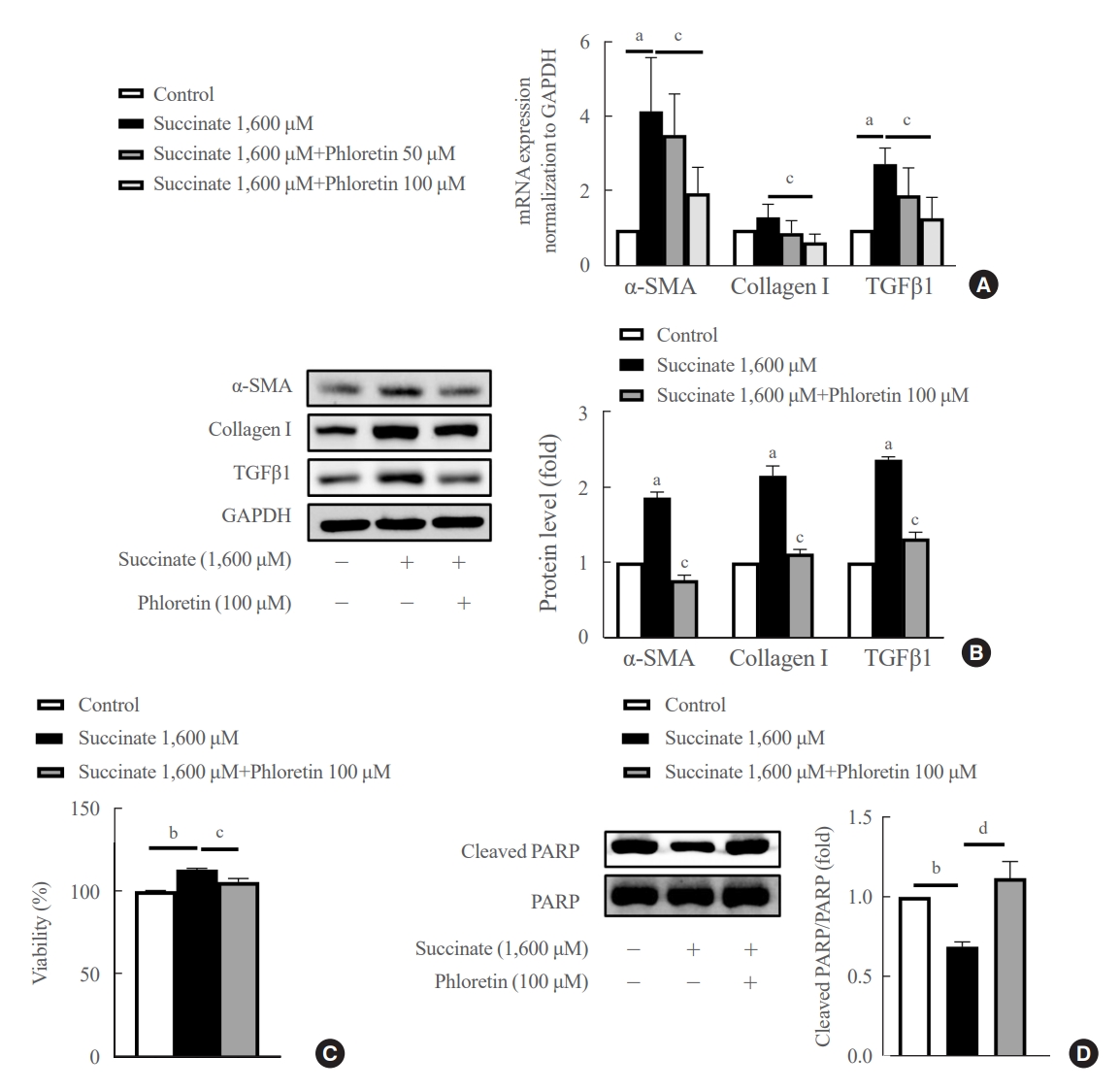
Hepatic stellate cells (HSCs) are the major cells which play a pivotal role in liver fibrosis. During injury, extracellular stimulators can induce HSCs transdifferentiated into active form. Phloretin showed its ability to protect the liver from injury, so in this research we would like to investigate the effect of phloretin on succinate-induced HSCs activation in vitro and liver fibrosis in vivo study.
Methods
In in vitro, succinate was used to induce HSCs activation, and then the effect of phloretin on activated HSCs was examined. In in vivo, succinate was used to generated liver fibrosis in mouse and phloretin co-treated to check its protection on the liver.
Results
Phloretin can reduce the increase of fibrogenic markers and inhibits the proliferation, migration, and contraction caused by succinate in in vitro experiments. Moreover, an upregulation of proteins associated with aerobic glycolysis occurred during the activation of HSCs, which was attenuated by phloretin treatment. In in vivo experiments, intraperitoneal injection of phloretin decreased expression of fibrotic and glycolytic markers in the livers of mice with sodium succinate diet-induced liver fibrosis. These results suggest that aerobic glycolysis plays critical role in activation of HSCs and succinate can induce liver fibrosis in mice, whereas phloretin has therapeutic potential for treating hepatic fibrosis.
Conclusion
Intraperitoneal injection of phloretin attenuated succinate-induced hepatic fibrosis and alleviates the succinate-induced HSCs activation.

- Diabetes, Obesity and Metabolism
- Stimulation of Alpha-1-Adrenergic Receptor Ameliorates Obesity-Induced Cataracts by Activating Glycolysis and Inhibiting Cataract-Inducing Factors
- Yong-Jik Lee, Yoo-Na Jang, Hyun-Min Kim, Yoon-Mi Han, Hong Seog Seo, Youngsub Eom, Jong-suk Song, Ji Hoon Jeong, Tae Woo Jung
- Endocrinol Metab. 2022;37(2):221-232. Published online March 23, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1237

- 3,658 View
- 137 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Obesity, the prevalence of which is increasing due to the lack of exercise and increased consumption of Westernized diets, induces various complications, including ophthalmic diseases. For example, obesity is involved in the onset of cataracts.
Methods
To clarify the effects and mechanisms of midodrine, an α1-adrenergic receptor agonist, in cataracts induced by obesity, we conducted various analytic experiments in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a rat model of obesity.
Results
Midodrine prevented cataract occurrence and improved lens clearance in OLETF rats. In the lenses of OLETF rats treated with midodrine, we observed lower levels of aldose reductase, tumor necrosis factor-α, and sorbitol, but higher levels of hexokinase, 5’-adenosine monophosphate-activated protein kinase-alpha, adenosine 5´-triphosphate, peroxisome proliferator-activated receptordelta, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, superoxide dismutase, and catalase.
Conclusion
The ameliorating effects of midodrine on cataracts in the OLETF obesity rat model are exerted via the following three mechanisms: direct inhibition of the biosynthesis of sorbitol, which causes cataracts; reduction of reactive oxygen species and inflammation; and (3) stimulation of normal aerobic glycolysis. -
Citations
Citations to this article as recorded by- α1-Adrenergic Receptors: Insights into Potential Therapeutic Opportunities for COVID-19, Heart Failure, and Alzheimer’s Disease
Dianne M. Perez
International Journal of Molecular Sciences.2023; 24(4): 4188. CrossRef - A new use for old drugs: identifying compounds with an anti-obesity effect using a high through-put semi-automated Caenorhabditis elegans screening platform
Freek Haerkens, Charlotte Kikken, Laurens Kirkels, Monique van Amstel, Willemijn Wouters, Els van Doornmalen, Christof Francke, Samantha Hughes
Heliyon.2022; 8(8): e10108. CrossRef
- α1-Adrenergic Receptors: Insights into Potential Therapeutic Opportunities for COVID-19, Heart Failure, and Alzheimer’s Disease

Review Article
- Miscellaneous
- Coordination of Multiple Cellular Processes by NR5A1/Nr5a1
- Ken-ichirou Morohashi, Miki Inoue, Takashi Baba
- Endocrinol Metab. 2020;35(4):756-764. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.402
- 5,039 View
- 159 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
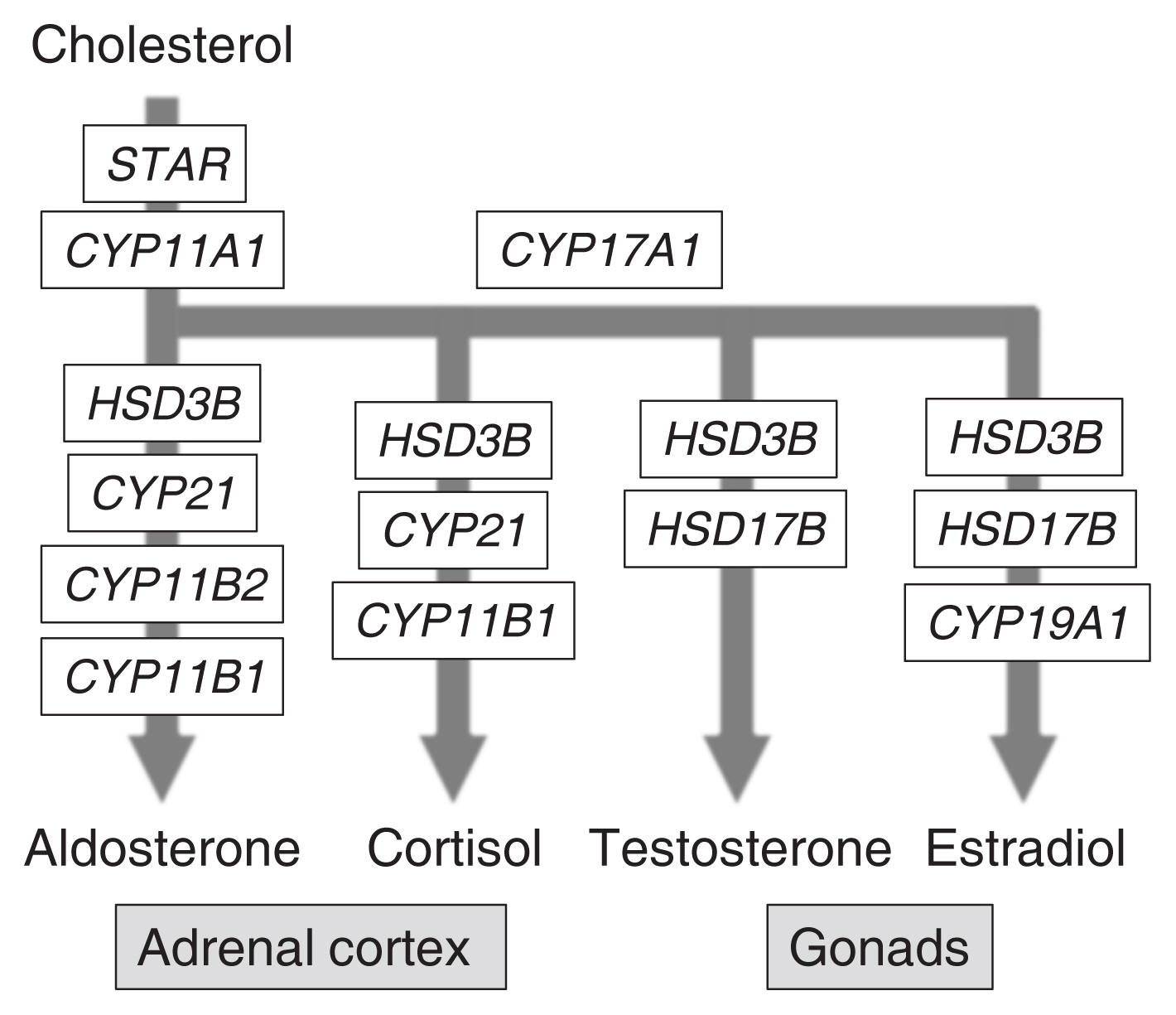
ePub - The agenesis of the gonads and adrenal gland in revealed by knockout mouse studies strongly suggested a crucial role for Nr5a1 (SF-1 or Ad4BP) in organ development. In relation to these striking phenotypes, NR5A1/Nr5a1 has the potential to reprogram cells to steroidogenic cells, endow pluripotency, and regulate cell proliferation. However, due to limited knowledge regarding NR5A1 target genes, the mechanism by which NR5A1/Nr5a1 regulates these fundamental processes has remained unknown. Recently, newlyestablished technologies have enabled the identification of NR5A1 target genes related to multiple metabolic processes, as well as the aforementioned biological processes. Considering that active cellular processes are expected to be accompanied by active metabolism, NR5A1 may act as a key factor for processes such as cell differentiation, proliferation, and survival by coordinating these processes with cellular metabolism. A complete and definite picture of the cellular processes coordinated by NR5A1/Nr5a1 could be depicted by accumulating evidence of the potential target genes through whole genome studies.
-
Citations
Citations to this article as recorded by- Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression
Fumiya Takahashi, Takashi Baba, Antonius Christianto, Shogo Yanai, Hyeon-Cheol Lee-Okada, Keisuke Ishiwata, Kazuhiko Nakabayashi, Kenichiro Hata, Tomohiro Ishii, Tomonobu Hasegawa, Takehiko Yokomizo, Man Ho Choi, Ken-ichirou Morohashi
Cell Reports.2024; 43(2): 113715. CrossRef - A novel heterozygous SF1/NR5A1 gene variant causes 46,XY DSD-gonadal dysgenesis with hypergonadotropic hypogonadism without adrenal insufficiency
Luis Ramos
Genes & Diseases.2024; 11(4): 101160. CrossRef - A conserved NR5A1-responsive enhancer regulates SRY in testis-determination
Denis Houzelstein, Caroline Eozenou, Carlos F. Lagos, Maëva Elzaiat, Joelle Bignon-Topalovic, Inma Gonzalez, Vincent Laville, Laurène Schlick, Somboon Wankanit, Prochi Madon, Jyotsna Kirtane, Arundhati Athalye, Federica Buonocore, Stéphanie Bigou, Gerard
Nature Communications.2024;[Epub] CrossRef - Testicular differentiation in 46,XX DSD: an overview of genetic causes
Maria Tereza Martins Ferrari, Elinaelma Suelane do Nascimento Silva, Mirian Yumie Nishi, Rafael Loch Batista, Berenice Bilharinho Mendonca, Sorahia Domenice
Frontiers in Endocrinology.2024;[Epub] CrossRef - Nuclear Receptor Gene Variants Underlying Disorders/Differences of Sex Development through Abnormal Testicular Development
Atsushi Hattori, Maki Fukami
Biomolecules.2023; 13(4): 691. CrossRef - Identification of a novel class of cortisol biosynthesis inhibitors and its implications in a therapeutic strategy for hypercortisolism
Soo Hyun Kim, Gi Hoon Son, Joo Young Seok, Sung Kook Chun, Hwayoung Yun, Jaebong Jang, Young-Ger Suh, Kyungjin Kim, Jong-Wha Jung, Sooyoung Chung
Life Sciences.2023; 325: 121744. CrossRef - Induced pluripotent stem cell line generated from a patient with differences in sex development (DSD) and multiple genetic variants including a large deletion in NR5A1
Aisha L. Siebert, Grace B. Schwartz, Hana Kubo, Monica M. Laronda
Stem Cell Research.2023; 71: 103154. CrossRef - Loss of NR5A1 in mouse Sertoli cells after sex determination changes cellular identity and induces cell death by anoikis
Sirine Souali-Crespo, Diana Condrea, Nadège Vernet, Betty Féret, Muriel Klopfenstein, Erwan Grandgirard, Violaine Alunni, Marie Cerciat, Matthieu Jung, Chloé Mayere, Serge Nef, Manuel Mark, Frédéric Chalmel, Norbert B. Ghyselinck
Development.2023;[Epub] CrossRef
- Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression


 KES
KES

 First
First Prev
Prev



