Search
- Page Path
- HOME > Search
Original Articles
- Diabetes, obesity and metabolism
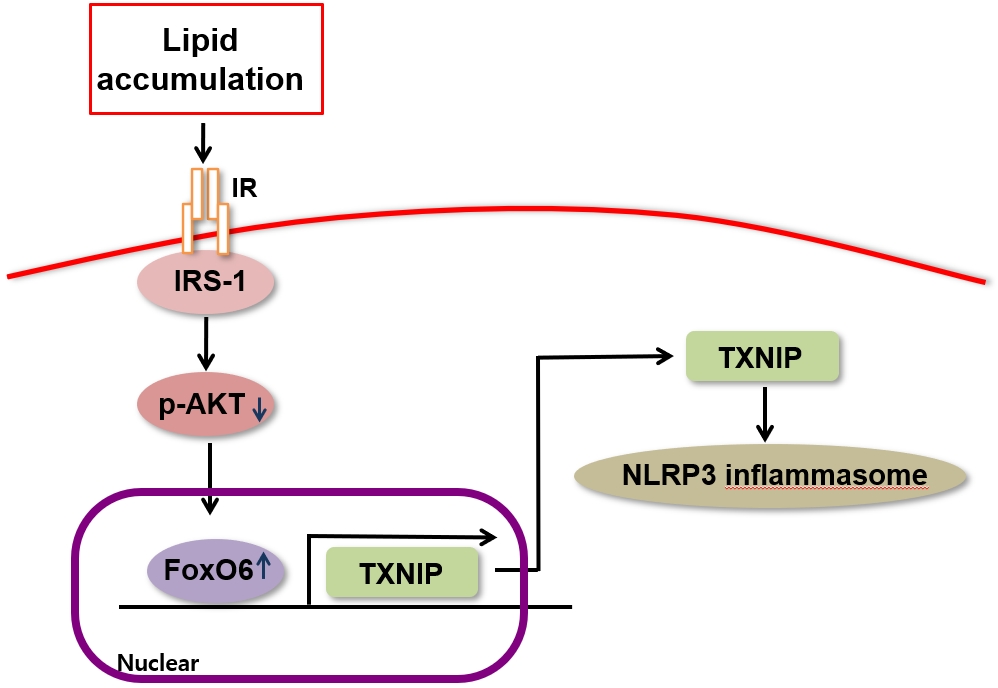
- FoxO6-Mediated TXNIP Induces Lipid Accumulation in the Liver through NLRP3 Inflammasome Activation
- Mi Eun Kim, Jun Sik Lee, Tae Won Kim, Min Hi Park, Dae Hyun Kim
- Endocrinol Metab. 2024;39(1):127-139. Published online February 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1826
- 1,162 View
- 45 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Hepatic steatosis, which involves the excessive accumulation of lipid droplets in hepatocytes, presents a significant global health concern due to its association with obesity and metabolic disorders. Inflammation plays a crucial role in the progression of hepatic steatosis; however, the precise molecular mechanisms responsible for this process remain unknown.
Methods
This study investigated the involvement of the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NLRP3) inflammasome and the forkhead box O6 (FoxO6) transcription factor in the pathogenesis of hepatic steatosis. We monitored the NLRP3 inflammasome and lipogenesis in mice overexpressing the constitutively active (CA)-FoxO6 allele and FoxO6-null mice. In an in vitro study, we administered palmitate to liver cells overexpressing CA-FoxO6 and measured changes in lipid metabolism.
Results
We administered palmitate treatment to clarify the mechanisms through which FoxO6 activates cytokine interleukin (IL)-1β through the NLRP3 inflammasome. The initial experiments revealed that dephosphorylation led to palmitate-induced FoxO6 transcriptional activity. Further palmitate experiments showed increased expression of IL-1β and the hepatic NLRP3 inflammasome complex, including adaptor protein apoptotic speck protein containing a caspase recruitment domain (ASC) and pro-caspase-1. Furthermore, thioredoxin-interacting protein (TXNIP), a key regulator of cellular redox conditions upstream of the NLRP3 inflammasome, was induced by FoxO6 in the liver and HepG2 cells.
Conclusion
The findings of this study shed light on the molecular mechanisms underpinning the FoxO6-NLRP3 inflammasome axis in promoting inflammation and lipid accumulation in the liver.

- Clinical Study
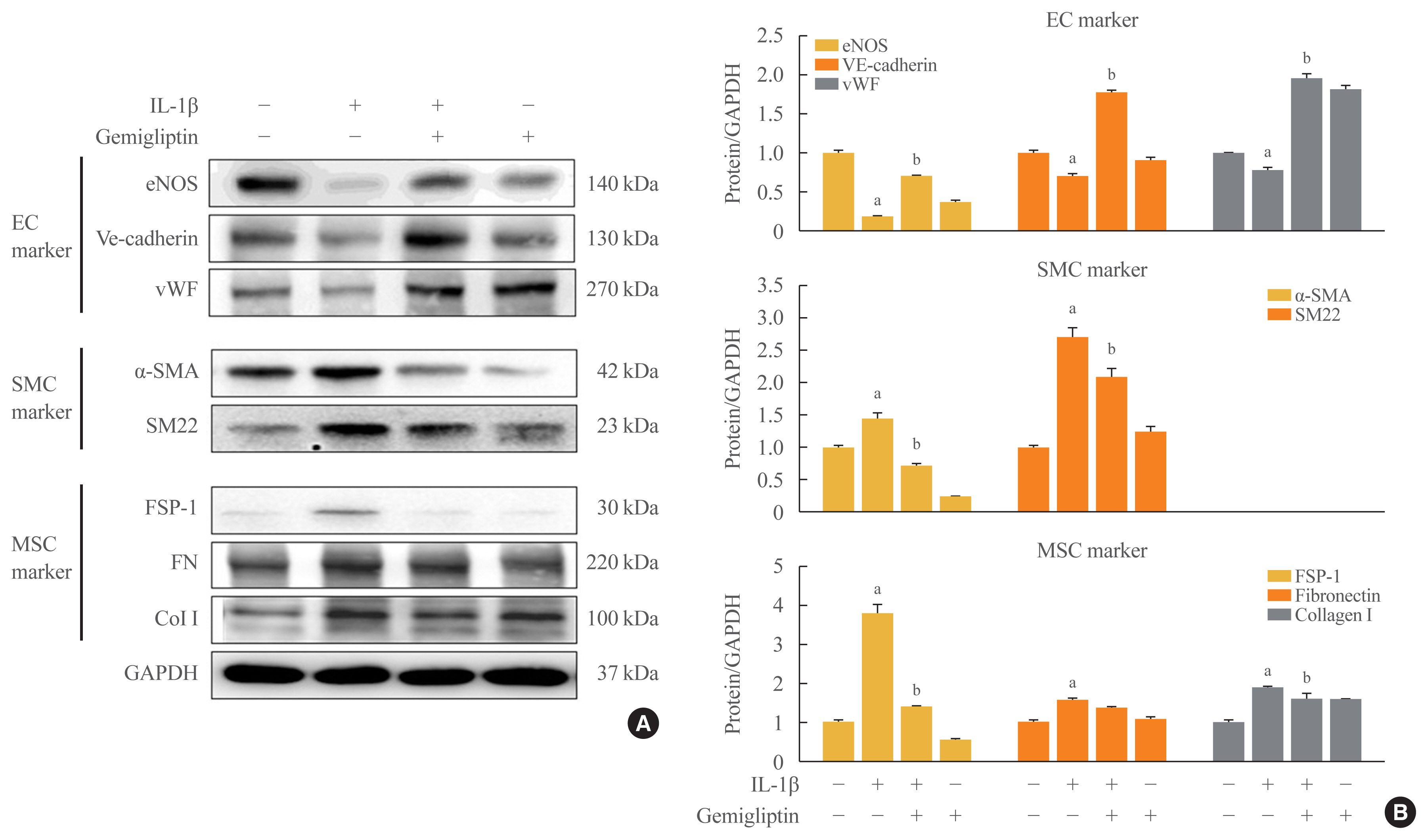
- Gemigliptin Inhibits Interleukin-1β–Induced Endothelial-Mesenchymal Transition via Canonical-Bone Morphogenetic Protein Pathway
- Oak-Kee Hong, Seong-Su Lee, Soon Jib Yoo, Min-Kyung Lee, Mee-Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
- Endocrinol Metab. 2020;35(2):384-395. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.384
- 6,778 View
- 139 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Endothelial-to-mesenchymal transition (EndMT) contributes to inflammatory conditions inducing conversion of endothelial cells (ECs) into activated fibroblasts, promoting fibrotic diseases. Pro-inflammatory cytokine is the most potent inducer of EndMT. We investigated inhibition of interleukin-1β (IL-1β)-induced EndMT by gemigliptin, a dipeptidyl peptidase-IV inhibitor.
Methods
We exposed human umbilical vein endothelial cells (HUVECs) to 10 ng/mL IL-1β/20 μM gemigliptin and analyzed the expression of endothelial, smooth muscle, mesenchymal, and osteoblastic markers, bone morphogenetic protein (BMP), Smad, and non-Smad signaling pathway proteins.
Results
Morphological changes showed gemigliptin blocked IL-1β-induced EndMT, upregulated EC markers, and downregulated smooth muscle and mesenchymal markers. IL-1β activation of HUVECs is initiated by the BMP/Smad and non-smad BMP signaling pathways. Gemigliptin inhibited IL-1β induction of BMP2 and 7, activin receptor type IA, BMP receptor type IA, and BMP receptor type II. Reversal of IL-1β-mediated inhibition of BMP-induced Smad1/5/8, Smad2, and Smad3 phosphorylation by gemigliptin suggests involvement of the Smad pathway in gemigliptin action. In the non-Smad BMP pathway, gemigliptin treatment significantly increased the deactivation of extracellular regulated protein kinase (ERK), p38, and JNK by IL-1β. Gemigliptin treatment suppressed BMP-2-induced expression of key osteoblastic markers including osterix, runt-related transcription factor 2, and hepcidin during IL-1β-induced EndMT.
Conclusion
We demonstrated a novel protective mechanism of gemigliptin against fibrosis by suppressing IL-1β-induced EndMT. -
Citations
Citations to this article as recorded by- Injured Endothelial Cell: A Risk Factor for Pulmonary Fibrosis
Weiming Zhao, Lan Wang, Yaxuan Wang, Hongmei Yuan, Mengxia Zhao, Hui Lian, Shuaichen Ma, Kai Xu, Zhongzheng Li, Guoying Yu
International Journal of Molecular Sciences.2023; 24(10): 8749. CrossRef - Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances
Zuxiang Yu, Chaoyu Xu, Bin Song, Shihao Zhang, Chong Chen, Changlong Li, Shuyu Zhang
Journal of Translational Medicine.2023;[Epub] CrossRef - MiRNAs in Systemic Sclerosis Patients with Pulmonary Arterial Hypertension: Markers and Effectors
Mor Zaaroor Levy, Noa Rabinowicz, Maia Yamila Kohon, Avshalom Shalom, Ariel Berl, Tzipi Hornik-Lurie, Liat Drucker, Shelly Tartakover Matalon, Yair Levy
Biomedicines.2022; 10(3): 629. CrossRef - Recent advance in treatment of atherosclerosis: Key targets and plaque-positioned delivery strategies
Li Li, Sainan Liu, Jianying Tan, Lai Wei, Dimeng Wu, Shuai Gao, Yajun Weng, Junying Chen
Journal of Tissue Engineering.2022; 13: 204173142210885. CrossRef - Vascular Calcification: New Insights Into BMP Type I Receptor A
Zhixing Niu, Guanyue Su, Tiantian Li, Hongchi Yu, Yang Shen, Demao Zhang, Xiaoheng Liu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Yi-Shen-Hua-Shi Granule Alleviates Adriamycin-Induced Glomerular Fibrosis by Suppressing the BMP2/Smad Signaling Pathway
Zhuojing Tan, Yachen Si, Yan Yu, Jiarong Ding, Linxi Huang, Ying Xu, Hongxia Zhang, Yihan Lu, Chao Wang, Bing Yu, Li Yuan
Frontiers in Pharmacology.2022;[Epub] CrossRef - Panax notoginseng Suppresses Bone Morphogenetic Protein-2 Expression in EA.hy926 Endothelial Cells by Inhibiting the Noncanonical NF-κB and Wnt/β-Catenin Signaling Pathways
Tsu-Ni Ping, Shu-Ling Hsieh, Jyh-Jye Wang, Jin-Bor Chen, Chih-Chung Wu
Plants.2022; 11(23): 3265. CrossRef - Vascular calcification: New insights into endothelial cells
Cheng Yuan, Lihua Ni, Changjiang Zhang, Xiaorong Hu, Xiaoyan Wu
Microvascular Research.2021; 134: 104105. CrossRef - Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model
Siwen Zhang, Qiyuan Chang, Pingping Li, Xiaoyu Tong, Yi Feng, Xinyao Hao, Xudong Zhang, Zhengwei Yuan, Jichun Tan
Nanoscale.2021; 13(15): 7334. CrossRef
- Injured Endothelial Cell: A Risk Factor for Pulmonary Fibrosis


 KES
KES

 First
First Prev
Prev



