Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(4); 2023 > Article
-
Original ArticleDiabetes, obesity and metabolism Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
 Keypoint
Keypoint
A study investigated the potential long-term impact of DPP-4 inhibitors on pancreatic carcinogenesis using data from the Korean National Health Insurance Service (2009-2012) on over 100,000 type 2 diabetes patients. Over roughly 8 years, there were 1,051 pancreatic cancer cases. No significant was found between DPP-4 inhibitor usage and pancreatic cancer risk. -
Mee Kyoung Kim1
 , Kyungdo Han2, Hyuk-Sang Kwon1, Soon Jib Yoo3
, Kyungdo Han2, Hyuk-Sang Kwon1, Soon Jib Yoo3
-
Endocrinology and Metabolism 2023;38(4):426-435.
DOI: https://doi.org/10.3803/EnM.2023.1737
Published online: July 20, 2023
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- Corresponding author: Soon Jib Yoo. Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 327 Sosa-ro, Wonmi-gu, Bucheon 14647, Korea Tel: +82-32-340-7011, Fax: +82-32-340-2669, E-mail: sjyoo@catholic.ac.kr
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The effects of dipeptidyl peptidase 4 (DPP-4) inhibitors over the course of long-term treatment remain unclear, and concerns have been raised regarding the role of DPP-4 inhibitors in carcinogenesis in the pancreas. Earlier studies of pancreatic adverse events have reported conflicting results.
-
Methods
- This study analyzed Korean National Health Insurance Service data from January 2009 to December 2012. Patients who had type 2 diabetes mellitus and took two or more oral glucose-lowering drugs (GLDs) were included. Patients prescribed DPP-4 inhibitors (n=51,482) or other GLDs (n=51,482) were matched at a 1:1 ratio using propensity score matching. The risk of pancreatic cancer was calculated using Kaplan-Meier curves and Cox proportional-hazards regression analysis.
-
Results
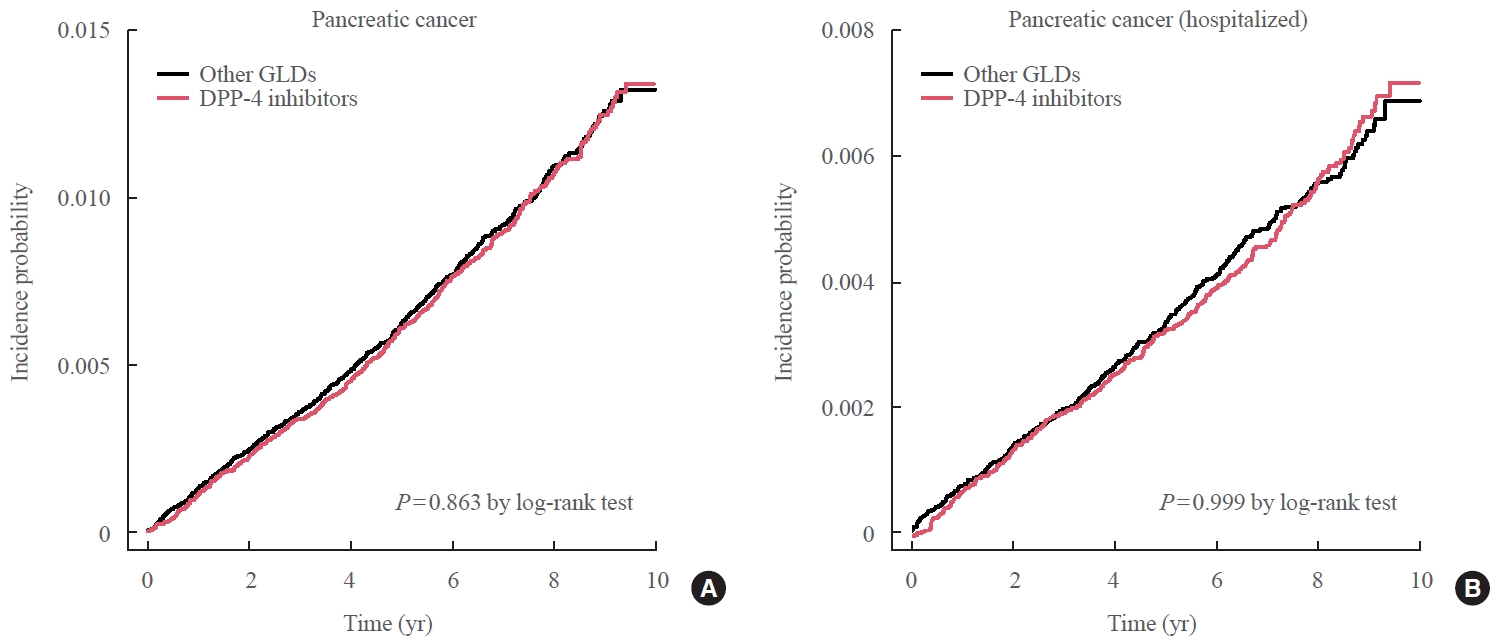
- During a median follow-up period of 7.95 years, 1,051 new cases of pancreatic cancer were identified. The adjusted hazard ratio (HR) for DPP-4 inhibitor use was 0.99 (95% confidence interval [CI], 0.88 to 1.12) compared with the other GLD group. In an analysis limited to cases diagnosed with pancreatic cancer during hospitalization, the adjusted HR for the use of DPP-4 inhibitors was 1.00 (95% CI, 0.86 to 1.17) compared with patients who took other GLDs. Using the other GLD group as the reference group, no trend was observed for elevated pancreatic cancer risk with increased DPP-4 inhibitor exposure.
-
Conclusion
- In this population-based cohort study, DPP-4 inhibitor use over the course of relatively long-term follow-up showed no significant association with an elevated risk of pancreatic cancer.
- There exists uncertainty regarding the effects of dipeptidyl peptidase 4 (DPP-4) inhibitors over the course of long-term follow-up. Concerns have also been expressed regarding the potential impact of DPP-4 inhibitors on carcinogenesis in the pancreas. Several observational studies and meta-analyses have investigated this issue [1-9], but with mutually inconsistent findings. In the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction (SAVOR-TIMI) 53 trial, saxagliptin use was not associated with significantly increased pancreatic cancer risk compared to placebo. Cancer events and cancer mortality occurred at similar proportions in the saxagliptin and placebo arms during follow-up (median, 2.1 years) [1]. In the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) study, fewer pancreatic cancer occurred in patients who received sitagliptin than in those who received placebo (9 [0.1%] vs. 14 [0.2%]; hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.28 to 1.51) [2]. However, those results should be interpreted cautiously because those trials had limited durations and reported few events. Furthermore, patients included in randomized controlled trials (RCTs) usually have higher levels of health than patients in the real-world and study cohorts. Furthermore, patients in RCTs are highly selected and reflect only a subset of the real-world population with type 2 diabetes mellitus (T2DM). Similarly, meta-analysis studies that include RCTs are limited by the characteristics of patients in these trials. An observational study showed an association between DPP-4 inhibitors and pancreatic cancer risk (HR, 1.81; 95% CI, 1.16 to 2.82) [3]. A cohort study found that incretin-based therapy had an adjusted HR of 2.14 for pancreatic cancer [4]. However, another observational study showed that DPP-4 inhibitor use was associated with a lower risk of pancreatic cancer than sulfonylurea use (HR, 0.6; 95% CI, 0.4 to 0.6) and exhibited an equivalent risk to that of thiazolidinediones (HR, 1.0; 95% CI, 0.7 to 1.4) [5].
- DPP-4 inhibitors first received regulatory approval in Korea in 2007; thus, they have now been in use for more than a decade. Accordingly, their safety in relation to pancreatic cancer can and should be studied in nationwide population-based cohorts with relatively long-term follow-up. This study analyzed data from the Korean National Health Insurance System (KNHIS) database with the aim of determining whether DPP-4 inhibitors are associated with pancreatic cancer risk in patients with T2DM. Since the KNHIS database contains representative data for the entire Korean population, it is suitable for conducting population-based nationwide research on T2DM in Korea.
INTRODUCTION
- Subjects
- A nonprofit organization, the KNHIS is the single insurer responsible for managing Korea’s health insurance system. KNHIS subscribers currently comprise approximately 97% of the Korean population, with the remainder being covered through Medical Aid. The Korean National Health Information Database (KNHID) has been extensively used by researchers [10-15] and includes an eligibility database (with information on type of eligibility, socioeconomic status, sex, and age), a medical treatment database (containing data from claims for medical expenses submitted by providers of medical services), a medical checkup database (with data on general health examinations and results from questionnaires about behavioral and lifestyle patterns), a medical care institution database (containing information on the number of physicians, equipment, location, and types of medical institutions), and death information. KNHIS enrollees are recommended to receive health checkups at least biennially [10,11].
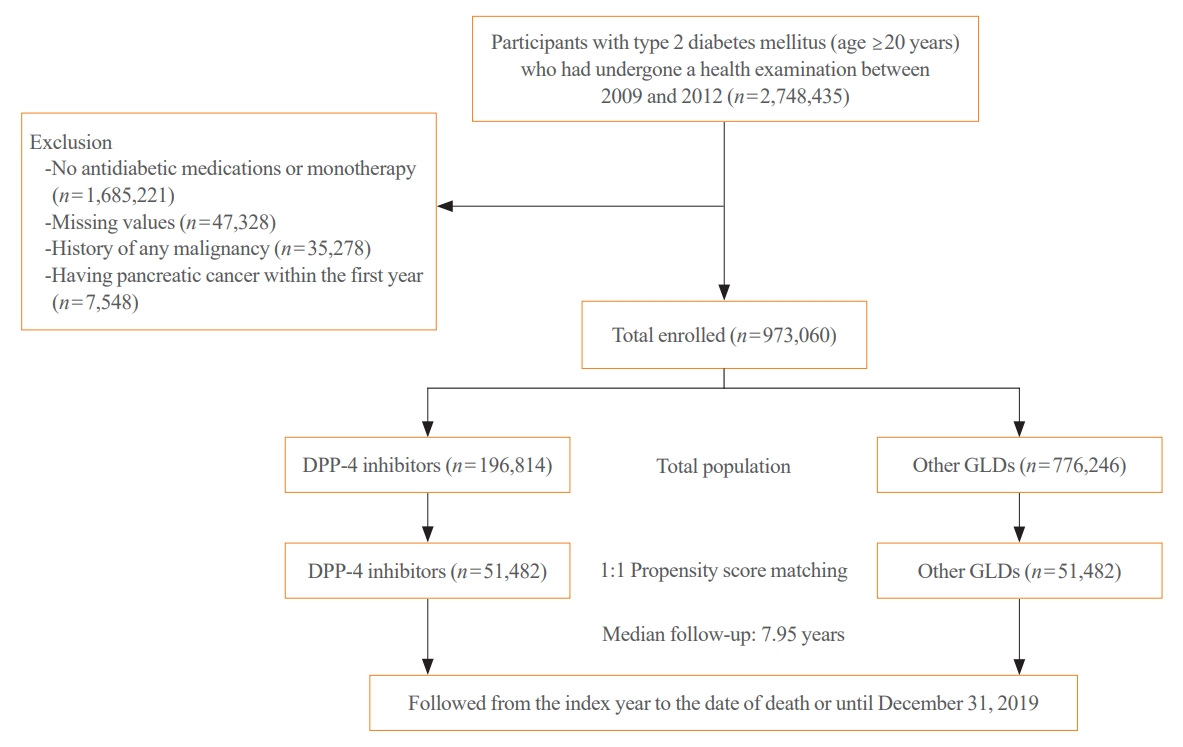
- The present study included data from 2,748,638 individuals ≥20 years of age who received a national health checkup between January 2009 and December 2012 (index year) and had T2DM. The definition of T2DM was a relevant International Classification of Diseases 10th Revision (ICD-10) code (E11–E14) and a prescription of antidiabetic medications, or a fasting blood glucose (FBG) concentration ≥126 mg/dL measured in the KNHIS health examination [10,11]. We only included patients with T2DM who took two or more oral glucose-lowering drugs (GLDs). A significant number of people either did not take antidiabetic medications after being diagnosed with T2DM or only used monotherapy (n=1,685,221). The reason for limiting the study population to patients taking multiple oral GLDs (n=1,063,214) was that DPP-4 inhibitors are recommended for use as second- to third-line treatments for T2DM [16]. We excluded subjects with any missing values (n=47,328), those with a history of any malignancy before the index year (n=35,278), and those with incident pancreatic cancer during the first year of follow-up (n=7,548) to avoid bias due to reverse causation. The final study population was 973,060 people with T2DM (Fig. 1). Of these, 196,814 used DPP-4 inhibitors and 776,246 used GLDs other than DPP-4 inhibitors. After performing 1:1 propensity score matching (PSM), 51,482 DPP-4 inhibitor users and 51,482 users of other GLDs remained. This study received approval from the Institutional Review Board of The Catholic University of Korea (No. SC22ZISE0176). The requirement for informed consent was waived due to the use of anonymized and deidentified information.
- Definition of covariates
- Data from the index year were used for the covariates, which included age, sex, socioeconomic status (income level), body mass index (kg/m2), current smoking status, alcohol consumption (with ≥30 g/day defined as heavy alcohol consumption), exercise (yes/no), and systolic and diastolic blood pressure (mm Hg). The health checkup program in the KNHIS involves anthropometric measurements, laboratory tests, and the administration of detailed lifestyle questionnaires [10,11]. Each participant filled out a self-reported health questionnaire at their health checkup. Blood samples were obtained following overnight fasting, and the serum creatinine, lipid, and FBG levels were quantified.
- Oral GLDs were classified as sulfonylureas, metformin, meglitinides, thiazolidinediones, DPP-4 inhibitors, and alpha-glucosidase inhibitors (AGIs). This study did not analyze glucagonlike peptide-1 (GLP-1) receptor agonists because they only became available in Korea after 2015. Prescription information (i.e., the drug class, date prescribed, days of supply, and quantity dispensed) was analyzed.
- Definition of primary outcome
- Since 2006, the KNHIS has utilized V-codes (i.e., special reimbursement codes) to reduce the copayment rate to 5% for intractable diseases, including cancer. Pancreatic cancer diagnoses must be physician-certified on the basis of clinical data for patients to benefit from this program [10-13]. The ICD-10 code C25 and the reimbursement code for cancer (V193) were used to identify incident pancreatic cancer cases. A sensitivity analysis was conducted that defined pancreatic cancer based on the recording of these two codes during hospitalization.
- To analyze the cumulative effect of DPP-4 inhibitors versus other GLDs, the medication possession ratio (MPR) was used [17,18], as defined below:
- The time interval (in days) from the index date to the first occurrence of the outcome event was defined as the time to outcome.
- Statistical analysis
- Categorical and continuous variables were reported as percentages and as mean±standard deviation or median (interquartile range), respectively. The main analyses were carried out after applying PSM to balance potential confounding factors between the groups, with the propensity score for each treatment group calculated via ordinary logistic regression with all the baseline covariates (other than oral GLDs) included in the Cox regression analysis. An acceptable difference in baseline characteristics was defined as an absolute standardized difference (ASD) of no more than 0.1 (10%). The potential effect modification by age (<65 years vs. ≥65 years), sex, use of insulin, number of oral GLDs (<3 and ≥3), diabetes mellitus (DM) duration (<5 and ≥5 years), use of sulfonylureas and the presence of cardiovascular disease or chronic kidney disease were evaluated via stratified analysis and interaction testing with the likelihood ratio test. In our analysis of the total population, pancreatic cancer risk was compared using Kaplan-Meier survival analysis with the log-rank test and multivariable-adjusted Cox hazard regression models based on the study population’s baseline characteristics. In the Cox regression analyses, we adjusted for all covariates in Table 1 except for oral GLDs. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), and statistical significance was defined using the threshold of a P value <0.05.
METHODS
- Population characteristics
- Before PSM, the DPP-4 inhibitor group was younger, had lower FBG and total cholesterol levels, and was less likely to have DM with a duration ≥5 years than the other GLD group. The mean ages of the other GLD and DPP-4 inhibitor groups were 60.8±10.5 and 57.6±10.7 years, respectively. The mean FBG levels of the other GLD and DPP-4 inhibitor groups were 144.1±51.3 and 139.2±46.4 mg/dL, respectively. The proportion of individuals taking three or more oral GLDs was 20.1% in the other GLD group and 17.8% in the DPP-4 inhibitor group.
- After 1:1 PSM, a matched cohort with 51,482 DPP-4 inhibitor users and 51,482 other GLD users was generated. Satisfactory balance (ASD <0.10) was found for all clinical characteristics (Table 1). We obtained the distribution of specific GLDs in the other GLD group (sulfonylureas, 82.4%; AGIs, 21.8%; thiazolidinediones, 18.4%; meglitinides, 4.5%). Metformin use was matched in both groups (ASD=0.089).
- Risk of pancreatic cancer according to DPP-4 inhibitor use in 51,482 PSM pairs
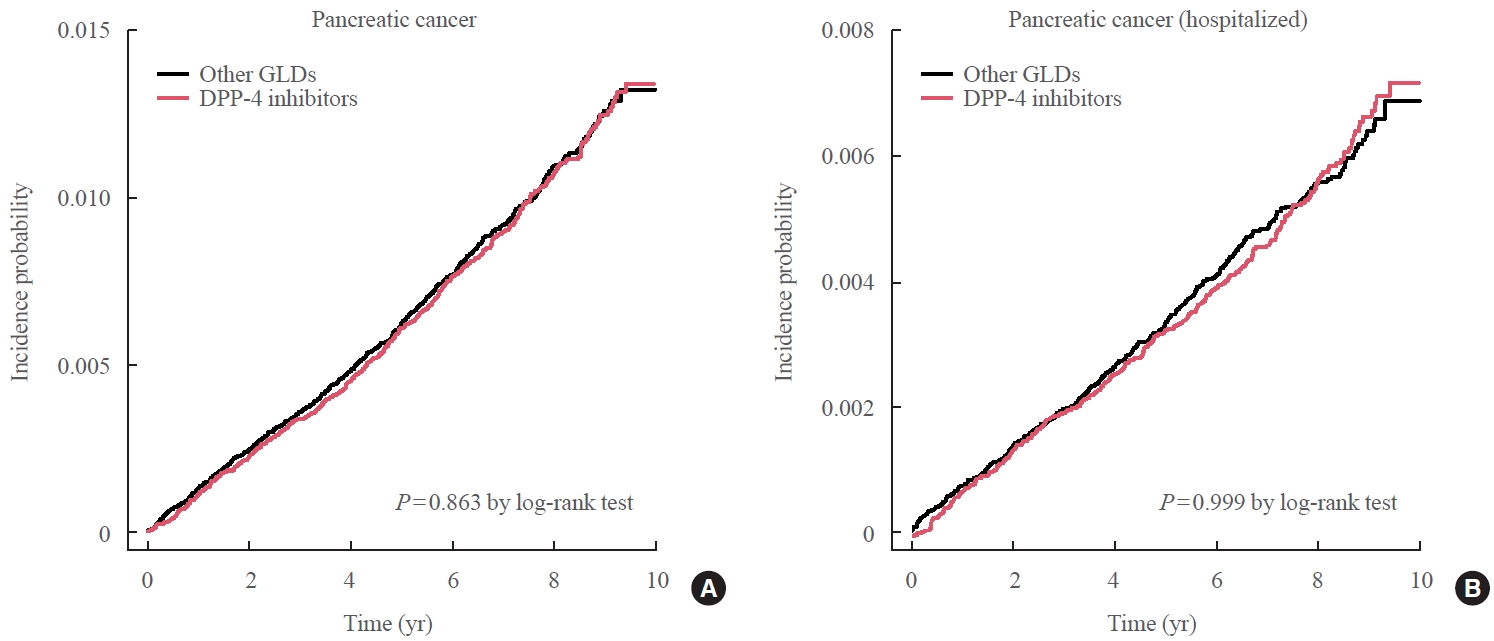
- During follow-up (median, 7.95 years), 1,051 new cases of pancreatic cancer were identified after excluding subjects who developed pancreatic cancer during the first year of follow-up. The incidence of pancreatic cancer was not significantly different between the two groups after PSM, demonstrating that DPP-4 inhibitor use showed no association with an elevated risk of pancreatic cancer compared with other GLDs (Fig. 2). The adjusted HR in the DPP-4 inhibitor group was 0.99 (95% CI, 0.88 to 1.12) compared to the other GLD group (Table 2). When the analysis was limited to cases diagnosed with pancreatic cancer during hospitalization, the adjusted HR in the DPP-4 inhibitor group was 1.00 (95% CI, 0.86 to 1.17) compared with the other GLD group (Table 3).
- Next, the risk of pancreatic cancer according to patients’ adherence to DPP-4 inhibitors was analyzed to assess the cumulative effect of DPP-4 inhibitor exposure on the risk of pancreatic cancer. Among DPP-4 inhibitor users, 55% had an MPR ≥80% and 27% had an MPR <50%. When the other GLD group was used as a reference, a higher MPR for DPP-4 inhibitors was not associated with a higher risk of pancreatic cancer (Tables 2, 3).
- Subgroup analysis
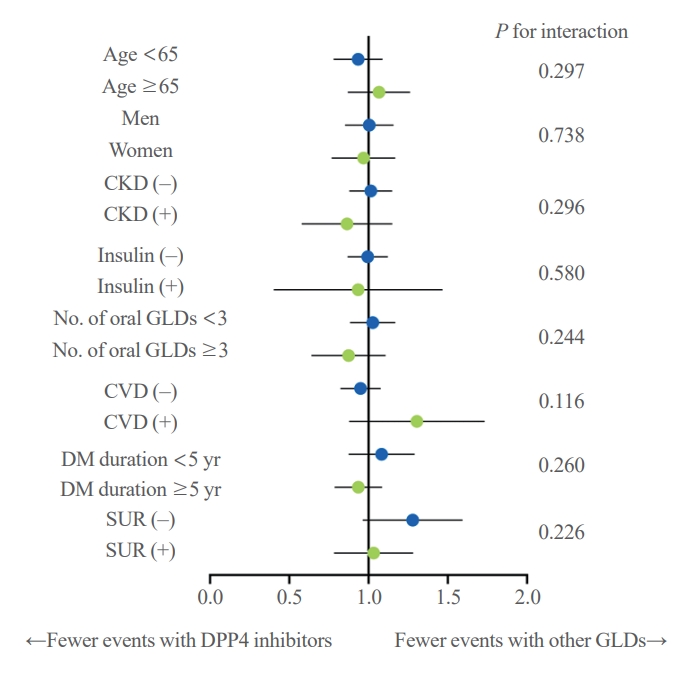
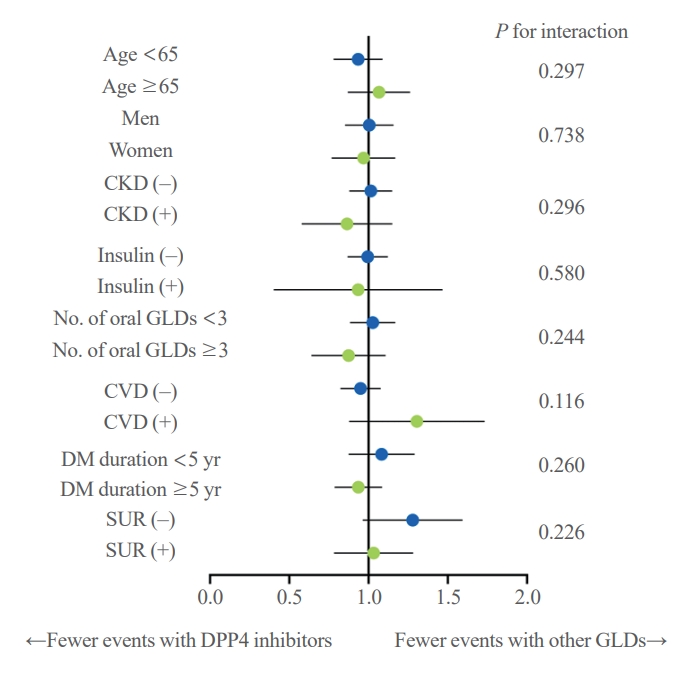
- The results showed no significant difference in the risk of pancreatic cancer between DPP-4 inhibitors and other GLDs groups across all subgroups (Fig. 3). The same results were seen in all subgroups when the pancreatic cancer diagnosis was confined to hospitalization (data not shown).
- Risk of pancreatic cancer according to DPP-4 inhibitor use: total population
- In the total study population, in which the median follow-up period was 8.1 years, 10,615 new cases of pancreatic cancer were identified after excluding subjects in whom pancreatic cancer developed during the first year of follow-up. In the overall population, DPP-4 inhibitor use did not show a significant association with a higher risk of pancreatic cancer. After adjusting for all covariates, including baseline characteristics, the adjusted HR was 1.02 (95% CI, 0.93 to 1.12) (Supplemental Table S1). Next, to assess the cumulative effect of drug exposure on the risk of pancreatic cancer, we analyzed pancreatic cancer risk according to patients’ adherence to DPP-4 inhibitors. Among DPP-4 inhibitor users, 68% had an MPR ≥80% and 17% had an MPR <50%. Using the other GLD group as a reference group, the MPR of DPP-4 inhibitors showed no significant association with pancreatic cancer risk (Supplemental Table S1).
RESULTS
- DPP-4 inhibitor use did not show a significant association with pancreatic cancer risk in this population-based cohort study. This finding remained consistent when restricting pancreatic cancer diagnoses to hospitalizations and when analyzing the total population before PSM. Moreover, the duration of exposure and adherence to DPP-4 inhibitors were not associated with pancreatic cancer risk. These are important findings regarding the safety of DPP-4 inhibitors, which are the second-most prescribed GLD in South Korea [14].
- Meta-analyses of RCTs have not confirmed the possibility of an association between DPP-4 inhibitor use and pancreatic cancer risk. The majority of RCTs included in those meta-analyses measured cancer as a post hoc outcome and had a short follow-up duration [1,2,6,7]. In addition, patients in clinical trials are generally healthier than real-world patients, making them less likely to develop cancer than real-world patients. The effects of drugs observed in RCTs often exceed their real-world effectiveness due to lower adherence to medication regimens in real-world patients, as well as the insufficient representativeness of RCT participants. DPP-4 inhibitors first received regulatory approval in Korea in 2007. In this context, observational studies have the advantage of including longer follow-up periods than RCTs, helping them to better capture long-term safety outcomes.
- According to a study using a sample cohort from the KNHIS that screened participants between 2007 and 2013, 35 cases were observed during exposure to DPP-4 inhibitors and 202 cases were observed during other anti-diabetes drug exposure [3]. Using a 6-month lag period for drug use, DPP-4 inhibitors were reported to be associated with elevated pancreatic cancer risk (HR, 1.81; 95% CI, 1.16 to 2.82) [3]. The authors pointed out that the possibility of reverse causality could not be ruled out, considering the absence of an increasing trend of pancreatic cancer with exposure duration, and limited follow-up (mean duration of follow-up, 3.6 years). In a previous study [3], only the ICD-10 code C25 was used to diagnose pancreatic cancer, with no use of V-codes. Previous research on the accuracy of ICD codes has concluded that caution should be exercised when interpreting administrative databases that rely solely on ICD codes [19]. The accuracy of using claims submitted for reimbursement purposes to identify patients with cancer is still questionable. Since 2006, the South Korean government has implemented a rare and intractable disease (RID) V-code registration program for 167 diseases, including cancers. Patients can register in the RID program if they are physician-certified as satisfying the diagnostic criteria, and registration makes them eligible for up to a 95% copayment reduction [10,11]. Medical institutions review these records before submission to the KNHIS because the KNHIS can refuse reimbursement if the diagnosis fails to satisfy certain criteria; thus, the diagnoses identified with V-codes have a high degree of reliability [10,11]. A previous study demonstrated the high accuracy of KNHID data gathered using the ICD-10 code and the V-code for pancreatic cancer, with overall sensitivity and specificity values of 99.95% and 98.7%, respectively [20]. Seo et al. [21] found that the overall and age-, sex-, and disease-specific cancer incidence rates were comparable between the KNHIS data and data from the National Cancer Registry of Korea. Their study also emphasized the usefulness of V-codes in the KNHIS database [21]. In our study, we applied both ICD-10 C25 and V-codes to define diagnoses pancreatic cancer. We also performed a sensitivity analysis limited to cases diagnosed during hospitalization. We excluded patients who developed pancreatic disease during the first year of follow-up, and exposure was lagged by 12 months to reduce the potential impact of reverse causality and to account for the latency period. Another advantage of this study is that it included more cases of pancreatic cancer than the above-cited meta-analyses [6,7]. The follow-up period in this study was 8 years, which is relatively long compared to previous studies.
- According to a study performed in Belgium and Italy, pancreatic cancer risk doubled shortly after newly prescribed incretinbased therapy [4]. These authors found that the risk of pancreatic cancer in individuals newly prescribed incretin therapy was 3.35 times higher (95% CI, 2.32 to 4.84) in the first three months after the first prescription, and then gradually decreased to 1.69 (95% CI, 1.12 to 2.55) 1 year after the first prescription. Based on the lack of a relationship between the duration of exposure and the risk of pancreatic cancer, the authors concluded that the protopathic bias would adequately explain their findings [4]. Measures of adherence can be used to estimate the cumulative effect of medications. Therefore, we used the MPR to analyze the risk of pancreatic cancer and found no statistically significant relationship. In our study, the incidence of pancreatic cancer per 1,000 person-years (PY) in patients with T2DM was 1.34. When the diagnosis of pancreatic cancer was defined as during hospitalization only, the incidence of pancreatic cancer per 1,000 PY in patients with T2DM was 0.81. This should be taken into account because we only included patients with T2DM who took two or more GLDs. In another study conducted in Korea, the incidence rate of pancreatic cancer per 1,000 PY in the diabetes group was 1.067, compared with 0.313 in the control, non-diabetic group [22]. Having DM was associated with an increased risk of developing pancreatic cancer (HR, 2.80; 95% CI, 2.31 to 3.40; P<0.001) [22]. Although incidence rates are not directly comparable between ethnic groups, Hispanic men and Asians have been reported to have a higher risk of diabetes-associated pancreatic cancer than Caucasians [23].
- Metformin use was matched in this study to avoid its associated confounding effects. Over 90% of all subjects were using metformin. In Korea, metformin was the most commonly used GLD (over 80%) during the study period (2009 to 2012), sulfonylureas were the second-most commonly used agents, and DPP-4 inhibitors were the third-most commonly used agents. Metformin use has been reported to have protective effects against colorectal, breast, and pancreatic cancer and is inversely associated with overall cancer morbidity and mortality [24,25]. However, no consensus exists regarding the role of sulfonylurea and insulin use in preventing malignancy, as observational studies have reported inconsistent findings (no association, reduced risk, or increased risk). Notably, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial (median follow-up, 6.2 years) reported no significant association of insulin glargine with overall and cancer-specific outcomes [26]. In our study, the most common comparator oral GLDs were sulfonylureas, followed in descending order by AGIs, thiazolidinediones, and meglitinides (Table 2).
- We acknowledge some limitations of this study. First, due to the observational nature of the study, we cannot rule out the possible existence of unmeasured confounders that could not be overcome by PSM. Confounders such as socioeconomic factors or other medical conditions not captured at baseline may have influenced both glucose-lowering medication selection and outcomes. Second, we did not consider patients’ history of pancreatitis, but instead adjusted for baseline characteristics such as smoking, alcohol drinking habits, obesity, and hypertriglyceridemia, which are important risk factors for pancreatitis. The diagnosis of pancreatitis through ICD-10 codes is known to be inaccurate in emergency departments and outpatient settings. Recently, GLP-1 receptor agonists and DPP-4 inhibitors have been shown to be associated with an elevated risk of cholecystitis [27,28]. A possible mechanism is that GLP-1 inhibits gallbladder motility and inhibits the secretion of cholecystokinin, which delays gallbladder emptying; alternatively, or that glucose-dependent insulinotropic polypeptide might impact gallbladder relaxation [27]. Because DPP-4 inhibitors are typically prescribed for longer periods of time in routine practice than in clinical trials, it may be particularly important to analyze events related to DPP-4 inhibitors using real-world clinical data. Third, we could not consider the glycemic control status during follow-up, which may be a possible confounder for the incidence of pancreatic cancer. We tried to balance baseline glycemic status by matching FBG levels, number of diabetes medications, and insulin use in comparison groups. Lastly, we could not take into account the duration of DPP-4 inhibitors use before index date. The DPP-4 inhibitors were introduced at the end of 2008 in Korea, then increased dramatically since 2009 [29]. Considering the timing of the introduction of DPP-4 inhibitors in Korea, the difference in duration of use before the index date is estimated to be less than 4 years.
- In conclusion, we have collected extensive data on the pancreatic cancer safety of DPP-4 inhibitors over the past decade. In this population-based cohort study with a relatively long follow-up, DPP-4 inhibitor use showed no association with an elevated risk of pancreatic cancer. Newer incretin-based therapies will also need to be studied for safety in terms of pancreatic cancer.
DISCUSSION
Supplementary Material
Supplemental Table S1.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: M.K.K., S.J.Y. Acquisition, analysis, or interpretation of data: K.H. Drafting the work or revising: M.K.K., H.S.K. Final approval of the manuscript: M.K.K., S.J.Y.
Article information
-
Acknowledgements
- This study was supported by Big Data Research Funds from the Korean Society of Endocrinology.
Values are expressed as mean±standard deviation, number (%), or median (interquartile range).
GLD, glucose-lowering drug; DPP-4, dipeptidyl peptidase 4; ASD, absolute standardized difference; BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; BP, blood pressure.
- 1. Leiter LA, Teoh H, Mosenzon O, Cahn A, Hirshberg B, Stahre CA, et al. Frequency of cancer events with saxagliptin in the SAVOR-TIMI 53 trial. Diabetes Obes Metab 2016;18:186–90.ArticlePubMed
- 2. Buse JB, Bethel MA, Green JB, Stevens SR, Lokhnygina Y, Aschner P, et al. Pancreatic safety of sitagliptin in the TECOS Study. Diabetes Care 2017;40:164–70.ArticlePubMedPMCPDF
- 3. Lee M, Sun J, Han M, Cho Y, Lee JY, Nam CM, et al. Nationwide trends in pancreatitis and pancreatic cancer risk among patients with newly diagnosed type 2 diabetes receiving dipeptidyl peptidase 4 inhibitors. Diabetes Care 2019;42:2057–64.ArticlePubMedPDF
- 4. Boniol M, Franchi M, Bota M, Leclercq A, Guillaume J, van Damme N, et al. Incretin-based therapies and the short-term risk of pancreatic cancer: results from two retrospective cohort studies. Diabetes Care 2018;41:286–92.ArticlePubMedPDF
- 5. Gokhale M, Buse JB, Gray CL, Pate V, Marquis MA, Sturmer T. Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 2014;16:1247–56.ArticlePubMedPMC
- 6. Dicembrini I, Montereggi C, Nreu B, Mannucci E, Monami M. Pancreatitis and pancreatic cancer in patientes treated with dipeptidyl peptidase-4 inhibitors: an extensive and updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2020;159:107981.ArticlePubMed
- 7. Overbeek JA, Bakker M, van der Heijden AA, van Herk-Sukel MP, Herings RM, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: a systematic review and meta-analysis. Diabetes Metab Res Rev 2018;34:e3004.ArticlePubMedPDF
- 8. Lee DY, Yu JH, Park S, Han K, Kim NH, Yoo HJ, et al. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: a nationwide population-based study in Korea. Sci Rep 2018;8:9719.ArticlePubMedPMCPDF
- 9. Montvida O, Green JB, Atherton J, Paul SK. Treatment with incretins does not increase the risk of pancreatic diseases compared to older anti-hyperglycaemic drugs, when added to metformin: real world evidence in people with type 2 diabetes. Diabet Med 2019;36:491–8.ArticlePubMedPDF
- 10. Kim MK, Han K, Lee SH. Current trends of big data research using the Korean National Health Information Database. Diabetes Metab J 2022;46:552–63.ArticlePubMedPMCPDF
- 11. Cho SW, Kim JH, Choi HS, Ahn HY, Kim MK, Rhee EJ. Big data research in the field of endocrine diseases using the Korean National Health Information Database. Endocrinol Metab (Seoul) 2023;38:10–24.ArticlePubMedPMCPDF
- 12. Han K, Kim B, Lee SH, Kim MK. A nationwide cohort study on diabetes severity and risk of Parkinson disease. NPJ Parkinsons Dis 2023;9:11.ArticlePubMedPMCPDF
- 13. Hong S, Kim KS, Han K, Park CY. Acromegaly and cardiovascular outcomes: a cohort study. Eur Heart J 2022;43:1491–9.ArticlePubMedPDF
- 14. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J 2022;46:417–26.ArticlePubMedPMCPDF
- 15. Kim D, Seo J, Ha KH, Kim DJ. Maintaining physical activity is associated with reduced major adverse cardiovascular events in people newly diagnosed with diabetes. J Obes Metab Syndr 2022;31:187–95.ArticlePubMedPMC
- 16. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J 2021;45:461–81.ArticlePubMedPMCPDF
- 17. Kim J, Han K, Kim B, Baek KH, Song KH, Kim MK, et al. Sodium-glucose cotransporter 2 inhibitors for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a nationwide propensity-score matched cohort study. Diabetes Res Clin Pract 2022;194:110187.ArticlePubMed
- 18. Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care 2004;27:2149–53.ArticlePubMedPDF
- 19. Yang MS, Park M, Back JH, Lee GH, Shin JH, Kim K, et al. Validation of cancer diagnosis based on the National Health Insurance Service Database versus the National Cancer Registry Database in Korea. Cancer Res Treat 2022;54:352–61.ArticlePubMedPMCPDF
- 20. Hwang YJ, Park SM, Ahn S, Lee JC, Park YS, Kim N. Accuracy of an administrative database for pancreatic cancer by international classification of disease 10th codes: a retrospective large-cohort study. World J Gastroenterol 2019;25:5619–29.ArticlePubMedPMC
- 21. Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev 2012;13:6163–8.ArticlePubMed
- 22. Lee HS, Chae W, Sung MJ, Keum J, Jo JH, Chung MJ, et al. Difference of risk of pancreatic cancer in new-onset diabetes and long-standing diabetes: a population-based cohort study. J Clin Endocrinol Metab 2023;108:1338–47.ArticlePubMedPDF
- 23. Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 2011;22:189–97.ArticlePubMedPMCPDF
- 24. Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012;107:620–6.ArticlePubMedPDF
- 25. Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:19–26.ArticlePubMed
- 26. Bordeleau L, Yakubovich N, Dagenais GR, Rosenstock J, Probstfield J, Chang Yu P, et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37:1360–6.ArticlePubMedPDF
- 27. He L, Wang J, Ping F, Yang N, Huang J, Li W, et al. Dipeptidyl peptidase-4 inhibitors and gallbladder or biliary disease in type 2 diabetes: systematic review and pairwise and network meta-analysis of randomised controlled trials. BMJ 2022;377:e068882.ArticlePubMedPMC
- 28. Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med 2016;176:1474–81.ArticlePubMed
- 29. Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e4018.PubMedPMC
References
Figure & Data
References
Citations

- Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef

 KES
KES

 PubReader
PubReader ePub Link
ePub Link Cite
Cite