Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(2); 2022 > Article
-
Original ArticleHypothalamus and Pituitary Gland Metabolic Impacts of Discontinuation and Resumption of Recombinant Human Growth Hormone Treatment during the Transition Period in Patients with Childhood-Onset Growth Hormone Deficiency
 Keypoint
Keypoint
This study investigated the metabolic changes associated with interrupting growth hormone (GH) treatment in 187 adolescents with childhood-onset growth hormone deficiency (CO-GHD) during the transition period treated at six academic centers in Korea. GH interruption during the transition period resulted in deterioration of metabolic parameters, and a longer duration of interruption was associated with worse outcomes. Therefore, early recommencement of GH should be considered in CO-GHD patients during their transition period. -
Yun Jeong Lee1
 , Yunha Choi2, Han-Wook Yoo2, Young Ah Lee1, Choong Ho Shin1, Han Saem Choi3, Ho-Seong Kim3, Jae Hyun Kim4, Jung Eun Moon5, Cheol Woo Ko5, Moon Bae Ahn6, Byung-Kyu Suh6, Jin-Ho Choi2
, Yunha Choi2, Han-Wook Yoo2, Young Ah Lee1, Choong Ho Shin1, Han Saem Choi3, Ho-Seong Kim3, Jae Hyun Kim4, Jung Eun Moon5, Cheol Woo Ko5, Moon Bae Ahn6, Byung-Kyu Suh6, Jin-Ho Choi2
-
Endocrinology and Metabolism 2022;37(2):359-368.
DOI: https://doi.org/10.3803/EnM.2021.1384
Published online: April 25, 2022
1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
2Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Departmend of Pediatrics, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea
4Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
5Department of Pediatrics, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
6Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding author: Jin-Ho Choi. Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-3991, Fax: +82-2-473-3725, E-mail: jhc@amc.seoul.kr
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Discontinuing growth hormone (GH) treatment during the transition to adulthood has been associated with adverse health outcomes in patients with childhood-onset growth hormone deficiency (CO-GHD). This study investigated the metabolic changes associated with interrupting GH treatment in adolescents with CO-GHD during the transition period.
-
Methods
- This study included 187 patients with CO-GHD who were confirmed to have adult GHD and were treated at six academic centers in Korea. Data on clinical parameters, including anthropometric measurements, metabolic profiles, and bone mineral density (BMD) at the end of childhood GH treatment, were collected at the time of re-evaluation for GHD and 1 year after treatment resumption.
-
Results
- Most patients (n=182, 97.3%) had organic GHD. The median age at treatment discontinuation and re-evaluation was 15.6 and 18.7 years, respectively. The median duration of treatment interruption was 2.8 years. During treatment discontinuation, body mass index Z-scores and total cholesterol, low-density lipoprotein, and non-high-density lipoprotein (HDL) cholesterol levels increased, whereas fasting glucose levels decreased. One year after GH treatment resumption, fasting glucose levels, HDL cholesterol levels, and femoral neck BMD increased significantly. Longer GH interruption (>2 years, 60.4%) resulted in worse lipid profiles at re-evaluation. The duration of interruption was positively correlated with fasting glucose and non-HDL cholesterol levels after adjusting for covariates.
-
Conclusion
- GH treatment interruption during the transition period resulted in worse metabolic parameters, and a longer interruption period was correlated with poorer outcomes. GH treatment should be resumed early in patients with CO-GHD during the transition period.
- Childhood-onset growth hormone deficiency (CO-GHD) is a pediatric-onset endocrine disorder that is associated with various health problems throughout life. Although the major role of growth hormone (GH) in children is to promote linear growth, it also exerts metabolic effects on multiple organs. These effects include enhancing anabolic protein metabolism, increasing bone mineral density (BMD), promoting lipolysis, and stimulating glucose metabolism [1,2]. Untreated patients with GHD have a high risk of developing cardiovascular disease [3,4], which is partially reversible with GH treatment [4,5].
- CO-GHD is a heterogeneous disease that is divided into two types: idiopathic GHD, which may resolve by the time children reach their final height, and organic GHD, which usually requires continuous GH treatment during the transition period and even in adulthood [6]. Because somatic maturation continues even after the cessation of physical growth in patients with organic CO-GHD, optimal management during the transition to adulthood is crucial [2,7]. Discontinuation of GH treatment after reaching the final height has been associated with abnormal body composition, reduced BMD, an adverse metabolic profile with increased cardiovascular risk, and impaired quality of life [8-11].
- Recent guidelines recommend that GH treatment should be continued until the final height is reached and resumed as early as possible after confirming adult GHD in patients with CO-GHD [12,13]. However, prolonged interruption of GH treatment between childhood and adulthood is frequently observed because of the high cost of treatment, lack of compliance, or recurrence of underlying diseases. Very few studies have explored metabolic derangements in patients with CO-GHD [14] and the adverse effects of prolonged treatment interruption in adolescents and young adults with CO-GHD in Korea [15].
- We hypothesized that the interruption of GH treatment during the transition period would worsen the metabolic parameters in patients with CO-GHD, and that the duration of treatment discontinuation would be correlated with the magnitude of these changes. This multi-center study evaluated the clinical and endocrinological changes related to the discontinuation and resumption of GH treatment in patients with CO-GHD during the transition period. The effects of treatment interruption on metabolic parameters in adolescents and young adults with COGHD were also investigated.
INTRODUCTION
- Patients
- Among patients diagnosed with CO-GHD (<18 years for boys and <16 years for girls at diagnosis) between 1994 and 2019 at six academic centers in Korea, 187 patients (99 boys and 88 girls) subsequently diagnosed with adult GHD and treated with recombinant human GH for >1 year were included in this study. Patients who were not diagnosed with GHD at retest and those with adult GHD who were treated for <1 year were excluded from this study (Supplemental Fig. S1).
- CO-GHD was diagnosed on the basis of a peak GH level of <10 μg/L on two separate GH stimulation tests using levodopa (125 to 500 mg) and insulin tolerance tests (regular insulin 0.1 U/kg) [13]. Patients underwent re-evaluation for GHD after they had reached their final height. Adult GHD was diagnosed on the basis of a peak GH level of <5 μg/L on insulin tolerance test [12,13]. The GH level was measured using a monoclonal immunoradiometric assay (IRMA, Diagnostics Systems Laboratories Inc., Webster, TX, USA).
- GH treatment during transition
- During childhood, all patients received recombinant human GH, which was discontinued when they reached their final height or when their growth velocity was <2 cm/year. GH treatment was discontinued in 27 patients before they reached their final height owing to financial constraints. Adult GHD was re-evaluated at least 1 month after GH treatment discontinuation when the patients reached their final height. After re-evaluation, patients with confirmed adult GHD received GH at a dose of 0.1 to 0.4 mg/day [12]. Patients were categorized into a long-gap group (treatment discontinuation duration >2 years) and a short-gap group (treatment discontinuation duration ≤2 years).
- Clinical and biochemical assessments
- Data on clinical parameters, including demographics, anthropometric measurements, and laboratory findings, were retrospectively collected at the end of childhood GH treatment, at re-evaluation, and 1 year after treatment resumption. Height, weight, and body mass index (BMI) Z-scores were determined on the basis of the 2017 Korean National Growth Charts [16]. Overweight and obesity in children and adolescents were defined as BMIs above the 85th and 95th percentiles of the age-and sex-matched reference data, respectively [17]. In adults, overweight and obesity were defined as BMIs of >23 and >25 kg/m2, respectively [18]. The laboratory parameters included the levels of fasting glucose, glycated hemoglobin (HbA1c), total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, insulin-like growth factor-1 (IGF-1), and IGF-binding protein-3 (IGFBP-3). Non-HDL cholesterol levels were calculated by subtracting the HDL cholesterol level from the total cholesterol level. For patients aged <19 years, dyslipidemia was defined as the presence of any of the following: total cholesterol ≥200 mg/dL, triglycerides ≥130 mg/dL, HDL cholesterol <40 mg/dL, and LDL cholesterol ≥130 mg/dL [19]; for those aged ≥19 years old, the cutoff values were as follows: total cholesterol ≥ 240 mg/dL, triglycerides ≥200 mg/dL, HDL cholesterol <40 mg/dL, and LDL cholesterol ≥160 mg/dL [20]. BMD was measured using dual-energy X-ray absorptiometry (DXA) scan with a Lunar Prodigy DXA system (General Electric Lunar Corporation, Madison, WI, USA) or a Hologic Discovery DXA system (Hologic Inc., Waltham, MA, USA). Z-scores for the lumbar spine and femoral neck were calculated according to the Korean reference values for children and adolescents [21,22].
- Statistical analysis
- Statistical analyses were performed using SPSS for Windows version 25.0 (IBM Corp., Armonk, NY, USA). All continuous variables were tested for normality and presented as mean±standard deviation or medians with interquartile ranges. The Student t test or the Mann-Whitney U test was used to compare continuous variables, and the chi-square test or Fisher exact test was used to compare categorical variables between the two groups. The paired t test, Wilcoxon signed-rank test, or McNemar test was used to compare variables among the following time points: at the end of GH treatment, at GH re-evaluation, and 1 year after treatment resumption. Linear regression analysis was performed to determine the relationship between the treatment interruption period and metabolic parameters, and multivariate models were adjusted for age, sex, BMI Z-scores, and peak GH levels at re-evaluation. After excluding patients with idiopathic GHD (n=5), statistical analyses were performed to evaluate the clinical changes of patients during GH treatment interruption and the effects of this interruption period on metabolic parameters. A P value of <0.05 was considered statistically significant.
- Ethical statement
- This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB number: 2019-1598). The requirement of informed consent was exempted because of the retrospective nature of the study, and anonymized clinical data were used in this study.
METHODS
- Clinical characteristics of the patients
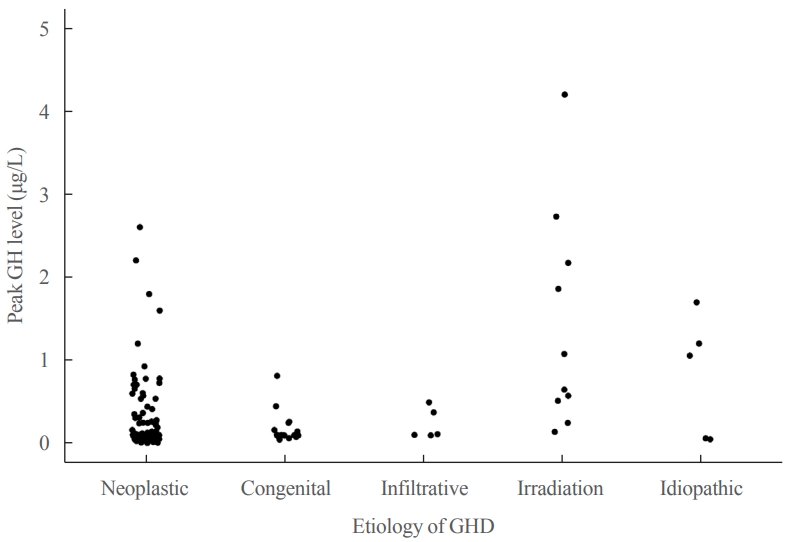
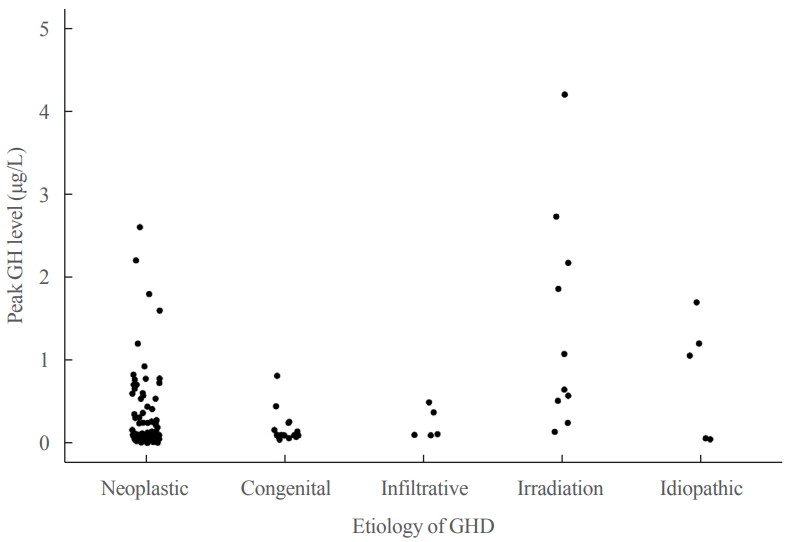
- Table 1 shows the underlying etiologies of CO-GHD. Most patients (n=182, 97.3%) had organic GHD, whereas five (2.7%) had idiopathic GHD. The most common cause of GHD was craniopharyngioma (n=83, 44.4%), followed by intracranial germ cell tumor (n=49, 26.2%), and congenital hypopituitarism (n=17, 9.1%). The mean age at the time of treatment discontinuation was 15.6±2.5 years, and the mean height, weight, and BMI Z-scores were −0.92, −0.70, and −0.53, respectively (Table 2). The median duration of treatment interruption was 2.8 years (range, 0.1 to 16.2), and GH treatment was discontinued in 113 (60.4%) patients for >2 years. The mean age at re-evaluation for GHD was 18.9±2.9 years. The mean peak GH levels at reevaluation were 0.30±0.54 μg/L (range, 0.01 to 4.2), and all patients except one had peak GH levels of <3 μg/L (Fig. 1). After re-evaluation, the patients received GH at a mean dose of 0.43±0.20 mg/day (0.01 mg/kg/day) for >1 year.
- GH treatment-associated changes in clinical characteristics during the transition period
- After the discontinuation of GH treatment, BMI Z-scores increased from 0.40 to 0.77 (P<0.001), whereas the IGF-1 and IGFBP-3 levels decreased significantly (P<0.001 for both) (Table 2). The fasting serum glucose levels decreased from 92.2 to 87.2 mg/dL (P=0.001), whereas there was not a significant change in the HbA1c levels. Total cholesterol, LDL cholesterol, and non-HDL cholesterol levels increased during the treatment interruption period (170.6 mg/dL vs. 184.7 mg/dL, P<0.001 for total cholesterol; 103.8 mg/dL vs. 121.3 mg/dL, P=0.016 for LDL cholesterol; 131.7 mg/dL vs. 144.2 mg/dL, P=0.045 for non-HDL cholesterol) (Table 2).
- After 1 year of GH treatment, the IGF-1 and IGFBP-3 levels increased significantly (P<0.001 for both). Moreover, BMI Z-scores continued to increase (P=0.002). The fasting glucose levels increased from 87.2 to 93.0 mg/dL (P=0.011), and HDL cholesterol levels increased from 43.7 to 47.2 mg/dL (P=0.032) without significant changes in other components of the lipid profile. The femoral neck BMD Z-score significantly increased from −1.10 to −0.90 after GH treatment (P=0.022). Statistical analyses excluding patients with idiopathic GHD revealed results similar to those of the analyses performed including all patients (Supplemental Table S1). The changes in clinical parameters at two time points are described in Supplemental Tables S2 (at the end of GH treatment and at the time of GH re-evaluation) and S3 (at the time of GH re-evaluation and 1 year after treatment resumption), which showed similar results as well.
- Effects of the treatment discontinuation duration on clinical characteristics
- When patients were compared on the basis of the duration of treatment interruption, no significant differences were noted in metabolic parameters between the long-gap (n=113) and the short-gap (n=74) groups at the end of GH treatment (Table 3). However, at re-evaluation, the long-gap group had worse lipid profiles with higher levels of total cholesterol (186.7 mg/dL vs. 175.7 mg/dL, P=0.030), triglycerides (163.9 mg/dL vs. 125.5 mg/dL, P=0.023), LDL cholesterol (120.6 mg/dL vs. 108.0 mg/dL, P=0.029), and non-HDL cholesterol (145.8 mg/dL vs. 127.1 mg/dL, P=0.002), and lower levels of HDL cholesterol (40.3 mg/dL vs. 47.1 mg/dL, P=0.004) (Table 3). After 1 year of GH treatment, the long-gap group still had higher BMI Z-scores than the short-gap group (P=0.046). Clinical characteristics before and after GH treatment were compared for each group. The results showed significant worsening of metabolic parameters in the long-gap group (Supplemental Table S4).
- In univariate regression analyses, a longer duration of GH treatment interruption was significantly correlated with increases in the levels of total cholesterol (β=1.95, P=0.018), LDL cholesterol (β=3.46, P=0.005), and non-HDL cholesterol (β=4.81, P<0.001). The duration of GH treatment interruption was inversely correlated with the levels of IGF-1 (β=−4.42, P=0.007), IGFBP-3 (β=−71.21, P=0.002), and HDL cholesterol (β=−1.06, P=0.036) at re-evaluation (Table 4). After adjusting for sex, age, BMI Z-scores, and peak GH levels, the interruption period was significantly correlated with increases in the level of fasting glucose (β=0.93, P=0.019) and non-HDL cholesterol (β=3.26, P=0.046) at re-evaluation. After adjusting for covariates, no significant associations were detected between the duration of treatment interruption and clinical parameters 1 year after treatment resumption (Supplemental Table S5). Statistical analyses performed including only patients with organic GHD revealed results consistent with those of analyses performed including all patients (Supplemental Tables S6, S7).
RESULTS
- In this multi-center study, most patients had organic GHD with low peak GH levels at re-evaluation. GH treatment was interrupted for >2 years in 60.4% of the patients. Consequently, they experienced an increase in BMI Z-scores with worsening lipid profiles after a median of 2.8 years of treatment discontinuation during the transition period; these changes did not resolve completely after 1 year of GH treatment. One year after resuming GH treatment, the HDL cholesterol levels and femoral neck BMD showed significant improvements. Patients with a longer duration of GH treatment interruption showed worse fasting glucose levels and lipid profiles at re-evaluation.
- In the present study, patients’ BMI Z-scores increased, whereas their IGF-1 and IGFBP-3 levels decreased during the GH treatment interruption period; however, their BMI Z-scores did not improve even after 1 year of GH replacement therapy. Several studies have reported that GH treatment discontinuation during the transition period led to an increase in BMI [23] and fat mass, in addition to a decrease in lean body mass [11,24,25]. Although the present study could not evaluate the body composition in detail, the increased BMI Z-scores in the cohort suggest that GH treatment discontinuation influences adiposity. Several trials have reported that GH replacement therapy during the transition period had a beneficial effect on body composition [8-11]; however, another study reported no changes in body composition after 2 years of treatment interruption [26]. A retrospective cohort study, similar to the present study, demonstrated a continual increase in BMI regardless of GH treatment during the transition period [27]. In the present study, the lack of improvement in BMI after resuming GH treatment might be attributed to the short treatment duration that was insufficient to overcome the effects of prolonged treatment interruption.
- Worsening of the lipid profile, with increased levels of total cholesterol, LDL cholesterol, and non-HDL cholesterol during GH cessation, was noted in this study; however, improvements in the levels of HDL cholesterol were observed after 1 year of GH replacement therapy. GH induces lipolysis in adipocytes and plays an important role in the regulation of lipoprotein metabolism [1]; therefore, adult GHD may result in dyslipidemia and abdominal fat deposition. As observed in the present study, other studies have noted that patients with CO-GHD often experience unfavorable changes in their lipid profiles, with increased LDL cholesterol and triglyceride levels after discontinuing GH treatment when they reach their final height [28-30]. GH replacement therapy during the transition period has been associated with decreased levels of total and LDL cholesterol in some studies [10,15,27], but not all [8,26,31]. This might be related to different durations of follow-up or the heterogeneous nature of CO-GHD.
- GH may enhance insulin sensitivity by improving body composition; however, it also stimulates glycogenolysis, gluconeogenesis, and lipolysis, thereby increasing blood glucose levels and inducing insulin resistance in the short term [32]. The patients in the present study had decreased fasting blood glucose levels after treatment discontinuation, which increased after treatment resumption. This is consistent with the findings of other studies [8,11,30]. Mild insulin resistance has been noted after short-term GH substitution in children and adults with GHD; however, the serum glucose levels remained within the normal range in most studies [5,33]. Long-term low-dose GH treatment may improve glucose metabolism in adult patients with GHD [3].
- Although BMD was evaluated in only a small number of patients in the present study, a significant improvement was observed in femoral neck BMD after GH treatment resumption. Several studies have reported the positive effects of GH replacement therapy on bone mass when patients with CO-GHD reached their final height [10,34,35]. Increased BMD was particularly apparent in the lumbar spine [10,34,35] but was also observed in the femoral neck [34,35]. However, the lack of changes in spine BMD and the lack of a control group in the present study made it difficult to confirm whether the observed improvement in femoral neck BMD was due to GH replacement therapy. Young adults with CO-GHD have been reported to show an initial temporary loss of BMD for several months after treatment resumption, followed by a subsequent increase in BMD [10,34]. This observation can be explained by a biphasic model of GH action, with an initial predominant bone resorption phase followed by increased bone formation [2]. Consequently, the net gain of bone mass usually takes >1 year, indicating the need for long-term follow-up to determine the real effects of GH treatment on BMD during the transition period. Attaining peak bone mass during the transition period is a crucial determinant of lifelong bone health [36], and further studies focusing on the long-term effects of GH treatment during the transition period are warranted.
- In the present study, patients with a longer duration of GH treatment interruption had worse metabolic profiles and lower levels of IGF-1 at re-evaluation. After adjustment for covariates, the duration of treatment interruption was noted to be significantly associated with increases in the levels of fasting blood glucose and non-HDL cholesterol. This suggests that long-term discontinuation of GH treatment results in compromised body composition and, consequently, worsens glucose and lipid metabolism. Longer GH treatment interruption has also been associated with a worse lipid profile or lower BMD in previous studies [29,37]. Although one clinical trial reported that at least 2 years of GH cessation was safe in adolescents with good metabolic status [26], the optimal shortest period of treatment discontinuation before re-evaluation has not been clarified [38].
- Current guidelines recommend re-evaluating patients for GHD at least 1 month after the discontinuation of pediatric GH treatment, particularly in patients with idiopathic isolated GHD. However, in patients with organic causes such as genetic or structural defects in the hypothalamic–pituitary region, re-evaluation is not required, and GH replacement therapy can be continued without interruption [12]. Although most patients in the present study had organic causes of GHD, the duration of GH treatment interruption was long (median, 2.8 years) because of the early discontinuation of GH treatment owing to the cost of treatment or lack of insurance coverage during the transition period. All patients except one showed peak GH levels of <3 μg/L in this study, which also supports the current recommendation that re-evaluation is not required in patients with organic causes of GHD [12]. The recent guidelines of the Korean Endocrine Society and Korean Society of Pediatric Endocrinology also recommend that GH replacement should be continued in patients with CO-GHD until they reach their final height and should be resumed as early as possible during the transition [13].
- This study has some limitations. First, the retrospective design of the study resulted in missing data on some clinical parameters such as fasting glucose and insulin levels, lipid profiles, family history, nutritional status, physical activity, adherence to GH treatment, and quality of life. Furthermore, this study evaluated neither the proportion of patients with adult GHD among those with CO-GHD nor the influence of other anterior pituitary hormone deficiencies. However, most patients had organic lesions with multiple pituitary hormone deficiencies, and the sensitivity analysis showed results consistent with those of the analysis including all patients. Second, this study did not include any control group without GH treatment. Finally, the duration of GH treatment after re-evaluation was short, and the long-term consequences of GH replacement therapy could not be evaluated. Thus, the effect of GH treatment on metabolic profiles might have been misinterpreted. Nevertheless, this is the first multi-center study to demonstrate the current treatment status and importance of GH treatment in patients with CO-GHD during the transition period in Korea. Because most of these patients were confirmed to have organic GHD, the study focused on the effects of GH treatment interruption in a relatively homogeneous group of patients.
- In conclusion, adolescents and young adults with CO-GHD exhibited worsened metabolic profiles after GH treatment interruption during the transition period, and these unfavorable changes were not fully reversible after 1 year of GH treatment. A longer duration of treatment discontinuation was associated with worse outcomes, and studies aiming at shortening this duration are warranted in the future.
DISCUSSION
Supplementary Information
Supplemental Table S1.
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
Supplemental Table S5.
Supplemental Table S6.
Supplemental Table S7.
Supplemental Fig. S1.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: C.H.S., J.H.C. Acquisition, analysis, or interpretation of data: Y.J.L., Y.C., H.W.Y., Y.A.L., C.H.S., H. S.C., H.S.K., J.H.K., J.E.M., C.W.K., M.B.A., B.K.S., J.H.C. Drafting the work or revising: Y.J.L., Y.C., J.H.C. Final approval of the manuscript: Y.J.L., Y.C., H.W.Y., Y.A.L., C.H.S., H.S.C., H.S.K., J.H.K., J.E.M., C.W.K., M.B.A., B.K.S., J.H.C.
Article information
-
Acknowledgements
- This work was supported by a grant (2019-04) from the Korean Society of Pediatric Endocrinology
| Variable |
At the end of GH treatment |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment interruption >2 years |
Treatment interruption ≤2 years |
Treatment interruption >2 years |
Treatment interruption ≤2 years |
Treatment interruption >2 years |
Treatment interruption ≤2 years |
|||||||
| No. | Value | No. | Value | No. | Value | No. | Value | No. | Value | No. | Value | |
| Body mass index, kg/m2 | 112 | 22.2±3.9 | 73 | 22.4±4.6 | 113 | 24.6±4.9a | 74 | 23.1±4.6a | 110 | 25.2±4.7 | 71 | 23.8±4.8 |
| Body mass index Z-score | 112 | 0.5±1.3 | 73 | 0.2±1.6 | 113 | 0.91±1.71 | 74 | 0.44±1.58 | 110 | 1.12±1.65a | 71 | 0.62±1.62a |
| IGF-1, μg/L | 91 | 317.6±246.7 | 64 | 311.1±198.1 | 113 | 69.3±49.8a | 74 | 94.6±85.5a | 112 | 163.5±102.4 | 69 | 172.8±136.8 |
| IGFBP-3, μg/L | 3,294.0±1,566.4a | 25 | 2,475.2±1,095.5a | 85 | 1,788.4±672.9 | 18 | 2,152.5±943.5 | 85 | 2,272.5±863.3 | 32 | 2,396.0±905.9 | |
| Fasting glucose, mg/dL | 85 | 93.0±12.4 | 44 | 90.4±11.7 | 109 | 87.8±13.2 | 70 | 87.3 ±10.4 | 97 | 94.7±27.5 | 52 | 91.9±13.4 |
| Glycated hemoglobin, % | 68 | 5.4±0.4 | 56 | 5.4±0.9 | 78 | 5.5±0.5 | 36 | 5.3±0.5 | 82 | 5.5±0.8 | 59 | 5.4±0.9 |
| Total cholesterol, mg/dL | 92 | 171.4±30.3 | 48 | 167.7±40.7 | 111 | 186.7±35.5a | 70 | 175.7±29.0a | 108 | 188.6±41.8 | 61 | 176.6±34.9 |
| Triglycerides, mg/dL | 63 | 146.9±77.9 | 20 | 125.0±66.8 | 101 | 163.9±137.0a | 62 | 125.5±76.7a | 88 | 165.3±93.8 | 40 | 138.4±70.8 |
| HDL cholesterol, mg/dL | 29 | 46.9±12.0 | 13 | 48.0±13.0 | 58 | 40.3±11.9b | 54 | 47.1±12.6b | 48 | 48.8±15.2 | 33 | 48.0±11.8 |
| LDL cholesterol, mg/dL | 29 | 102.5±27.6 | 13 | 103.8±30.9 | 58 | 120.6±35.2a | 54 | 108.0±24.2a | 48 | 112.5±34.8 | 33 | 110.5±26.7 |
| Non-HDL cholesterol, mg/dL | 29 | 133.3±25.9 | 13 | 123.8±25.8 | 58 | 145.8±33.1b | 54 | 127.1±27.7b | 48 | 137.7±37.4 | 33 | 131.8±24.8 |
| Lumbar spine BMD Z-score | - | - | - | - | 54 | −1.4 (−2.4 to −0.8) | 6 | −1.7 (−2.7 to −0.2) | 31 | −1.6 (−2.4 to −0.7) | 8 | −1.7 (−2.8 to −1.6) |
| Femoral neck BMD Z-score | - | - | - | - | 43 | −1.0 (−1.8 to −0.3) | 6 | −1.6 (−2.1 to 0.2) | 29 | −0.9 (−1.2 to −0.1) | 8 | −0.9 (−1.7 to 0.2) |
| Outcome variable | Unadjusted, β (95% CI) | Adjusted, β (95% CI)c |
|---|---|---|
| Body mass index, kg/m2 | 0.12 (−0.11 to 0.35) | −0.03 (−0.33 to 0.27) |
| Body mass index Z-score | 0.04 (−0.04 to 0.12) | −0.01 (−0.10 to 0.08) |
| IGF-1, μg/L | −4.42 (−7.60 to −1.25)b | −2.19 (−5.95 to 1.57) |
| IGFBP-3, μg/L | −71.21 (−114.18 to −28.23)b | −52.00 (−105.16 to 1.16) |
| Fasting glucose, mg/dL | 0.53 (−0.06 to 1.12) | 0.93 (0.16 to 1.70)a |
| Glycated hemoglobin, % | 0.01 (−0.02 to 0.04) | 0.00 (−0.04 to 0.04) |
| Total cholesterol, mg/dL | 1.95 (0.35 to 3.55)a | 1.44 (-0.69 to 3.57) |
| Triglycerides, mg/dL | 4.80 (−1.09 to 10.69) | 4.13 (−3.84 to 12.09) |
| HDL cholesterol, mg/dL | −1.06 (−2.03 to −0.08)a | −0.79 (−2.10 to 0.51) |
| LDL cholesterol, mg/dL | 3.46 (1.11 to 5.81)b | 2.00 (−1.14 to 5.14) |
| Non-HDL cholesterol, mg/dL | 4.81 (2.46 to 7.16)b | 3.26 (0.10 to 6.42)a |
GH, growth hormone; CI, confidence interval; IGF-1, insulin-like growth factor-1; IGFBP-3, IGF-binding protein-3; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a P<0.05;
b P<0.01;
c Models were adjusted for age, sex, peak growth hormone levels at re-evaluation for body mass index and body mass index Z-scores; the models were adjusted for age, sex, body mass index Z-scores, and peak GH levels for other parameters.
- 1. Rothermel J, Reinehr T. Metabolic alterations in paediatric GH deficiency. Best Pract Res Clin Endocrinol Metab 2016;30:757–70.ArticlePubMed
- 2. Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev 1998;19:55–79.ArticlePubMed
- 3. Gazzaruso C, Gola M, Karamouzis I, Giubbini R, Giustina A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH: an update. J Clin Endocrinol Metab 2014;99:18–29.ArticlePubMed
- 4. Capalbo D, Mattace Raso G, Esposito A, Di Mase R, Barbieri F, Meli R, et al. Cluster of cardiometabolic risk factors in children with GH deficiency: a prospective, case-control study. Clin Endocrinol (Oxf) 2014;80:856–62.ArticlePubMed
- 5. Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P, et al. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 2004;89:2192–9.ArticlePubMed
- 6. Yuen K, Alter CA, Miller BS, Gannon AW, Tritos NA, Samson SL, et al. Adult growth hormone deficiency: optimizing transition of care from pediatric to adult services. Growth Horm IGF Res 2021;56:101375.ArticlePubMed
- 7. Sbardella E, Pozza C, Isidori AM, Grossman AB. Endocrinology and adolescence: dealing with transition in young patients with pituitary disorders. Eur J Endocrinol 2019;181:R155–71.ArticlePubMed
- 8. Carroll PV, Drake WM, Maher KT, Metcalfe K, Shaw NJ, Dunger DB, et al. Comparison of continuation or cessation of growth hormone (GH) therapy on body composition and metabolic status in adolescents with severe GH deficiency at completion of linear growth. J Clin Endocrinol Metab 2004;89:3890–5.ArticlePubMed
- 9. Attanasio AF, Shavrikova E, Blum WF, Cromer M, Child CJ, Paskova M, et al. Continued growth hormone (GH) treatment after final height is necessary to complete somatic development in childhood-onset GH-deficient patients. J Clin Endocrinol Metab 2004;89:4857–62.ArticlePubMed
- 10. Underwood LE, Attie KM, Baptista J; Genentech Collaborative Study Group. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a twoyear, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab 2003;88:5273–80.ArticlePubMed
- 11. Vahl N, Juul A, Jorgensen JO, Orskov H, Skakkebaek NE, Christiansen JS. Continuation of growth hormone (GH) replacement in GH-deficient patients during transition from childhood to adulthood: a two-year placebo-controlled study. J Clin Endocrinol Metab 2000;85:1874–81.ArticlePubMed
- 12. Yuen K, Biller B, Radovick S, Carmichael JD, Jasim S, Pantalone KM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr Pract 2019;25:1191–232.ArticlePubMed
- 13. Kim JH, Chae HW, Chin SO, Ku CR, Park KH, Lim DJ, et al. Diagnosis and treatment of growth hormone deficiency: a position statement from Korean Endocrine Society and Korean Society of Pediatric Endocrinology. Endocrinol Metab (Seoul) 2020;35:272–87.ArticlePubMedPMC
- 14. Lim HH, Kang MJ, Yun IS, Lee YA, Shin CH, Yang SW. Prevalence and risk factors of the metabolic syndrome in young adults with childhood-onset hypopituitary growth hormone deficiency. Korean J Pediatr 2010;53:892–7.ArticlePubMedPMC
- 15. Kim JH, Cho JH, Yoo HW, Choi JH. Efficacy of growth hormone therapy in adults with childhood-onset growth hormone deficiency. Ann Pediatr Endocrinol Metab 2014;19:32–5.ArticlePubMedPMC
- 16. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr 2018;61:135–49.ArticlePubMedPMC
- 17. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–57.ArticlePubMedPMC
- 18. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr 2021;30:81–92.ArticlePubMedPMC
- 19. Lim JS, Kim EY, Kim JH, Yoo JH, Yi KH, Chae HW, et al. 2017 Clinical practice guidelines for dyslipidemia of Korean children and adolescents. Ann Pediatr Endocrinol Metab 2020;25:199–207.ArticlePubMedPMC
- 20. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia. Korean J Intern Med 2019;34:723–71.ArticlePubMedPMC
- 21. Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, et al. Bone mineral density according to age, bone age, and pubertal stages in Korean children and adolescents. J Clin Densitom 2010;13:68–76.ArticlePubMed
- 22. Kang MJ, Hong HS, Chung SJ, Lee YA, Shin CH, Yang SW. Body composition and bone density reference data for Korean children, adolescents, and young adults according to age and sex: results of the 2009-2010 Korean National Health and Nutrition Examination Survey (KNHANES). J Bone Miner Metab 2016;34:429–39.ArticlePubMed
- 23. Bazarra-Castro MA, Sievers C, Schwarz HP, Pozza SB, Stalla GK. Changes in BMI and management of patients with childhood onset growth hormone deficiency in the transition phase. Exp Clin Endocrinol Diabetes 2012;120:507–10.ArticlePubMed
- 24. Binder G, Donner J, Becker B, Bauer JL, Schweizer R. Changes in body composition in male adolescents with childhood-onset GH deficiency during transition. Clin Endocrinol (Oxf) 2019;91:432–9.ArticlePubMed
- 25. Boot AM, van der Sluis IM, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density and body composition in adolescents with childhood-onset growth hormone deficiency. Horm Res 2009;71:364–71.ArticlePubMed
- 26. Mauras N, Pescovitz OH, Allada V, Messig M, Wajnrajch MP, Lippe B, et al. Limited efficacy of growth hormone (GH) during transition of GH-deficient patients from adolescence to adulthood: a phase III multicenter, double-blind, randomized two-year trial. J Clin Endocrinol Metab 2005;90:3946–55.ArticlePubMed
- 27. Courtillot C, Baudoin R, Du Souich T, Saatdjian L, Tejedor I, Pinto G, et al. Monocentric study of 112 consecutive patients with childhood onset GH deficiency around and after transition. Eur J Endocrinol 2013;169:587–96.ArticlePubMed
- 28. Rothermel J, Lass N, Bosse C, Reinehr T. Impact of discontinuation of growth hormone treatment on lipids and weight status in adolescents. J Pediatr Endocrinol Metab 2017;30:749–57.ArticlePubMed
- 29. Koltowska-Haggstrom M, Geffner ME, Jonsson P, Monson JP, Abs R, Hana V, et al. Discontinuation of growth hormone (GH) treatment during the transition phase is an important factor determining the phenotype of young adults with nonidiopathic childhood-onset GH deficiency. J Clin Endocrinol Metab 2010;95:2646–54.ArticlePubMed
- 30. Colao A, Di Somma C, Salerno M, Spinelli L, Orio F, Lombardi G. The cardiovascular risk of GH-deficient adolescents. J Clin Endocrinol Metab 2002;87:3650–5.ArticlePubMed
- 31. Camtosun E, Siklar Z, Berberoglu M. Prospective follow-up of children with idiopathic growth hormone deficiency after termination of growth hormone treatment: is there really need for treatment at transition to adulthood? J Clin Res Pediatr Endocrinol 2018;10:247–55.ArticlePubMedPMC
- 32. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152–77.ArticlePubMed
- 33. Ciresi A, Giordano C. Glucose metabolism in children with growth hormone deficiency. Front Endocrinol (Lausanne) 2018;9:321.ArticlePubMedPMC
- 34. Conway GS, Szarras-Czapnik M, Racz K, Keller A, Chanson P, Tauber M, et al. Treatment for 24 months with recombinant human GH has a beneficial effect on bone mineral density in young adults with childhood-onset GH deficiency. Eur J Endocrinol 2009;160:899–907.ArticlePubMed
- 35. Shalet SM, Shavrikova E, Cromer M, Child CJ, Keller E, Zapletalova J, et al. Effect of growth hormone (GH) treatment on bone in postpubertal GH-deficient patients: a 2-year randomized, controlled, dose-ranging study. J Clin Endocrinol Metab 2003;88:4124–9.ArticlePubMed
- 36. Kralick AE, Zemel BS. Evolutionary perspectives on the developing skeleton and implications for lifelong health. Front Endocrinol (Lausanne) 2020;11:99.ArticlePubMedPMC
- 37. Tritos NA, Hamrahian AH, King D, Greenspan SL, Cook DM, Jonsson PJ, et al. A longer interval without GH replacement and female gender are associated with lower bone mineral density in adults with childhood-onset GH deficiency: a KIMS database analysis. Eur J Endocrinol 2012;167:343–51.ArticlePubMedPMC
- 38. Ahmid M, Ahmed SF, Shaikh MG. Childhood-onset growth hormone deficiency and the transition to adulthood: current perspective. Ther Clin Risk Manag 2018;14:2283–91.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Ghrelin regulating liver activity and its potential effects on liver fibrosis and Echinococcosis
Jiang Zhu, Tanfang Zhou, Meng Menggen, Kalibixiati Aimulajiang, Hao Wen
Frontiers in Cellular and Infection Microbiology.2024;[Epub] CrossRef - Relationship between the Stimulated Peak Growth Hormone Level and Metabolic Parameters in Children with Growth Hormone Deficiency
Seong Yong Lee
The Ewha Medical Journal.2023;[Epub] CrossRef - Dyslipidaemia and growth hormone deficiency – A comprehensive review
Matthias Hepprich, Fahim Ebrahimi, Emanuel Christ
Best Practice & Research Clinical Endocrinology & Metabolism.2023; 37(6): 101821. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite