Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(2); 2024 > Article
-
Original ArticleThyroid Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
 Keypoint
Keypoint
· This study assessed the cost-effectiveness of early screening versus symptomatic detection for thyroid cancer in Korea.
· The findings suggest that early screening is cost-effective, with an incremental cost-effectiveness ratio of $7133 per quality-adjusted life year gained.
· Early detection and subsequent lobectomy contribute to the cost-effectiveness of early screening, while symptomatic detection at an advanced stage makes early screening more cost-effective. -
Han-Sang Baek1
 , Jeonghoon Ha2, Kwangsoon Kim3, Ja Seong Bae3, Jeong Soo Kim3, Sungju Kim4, Dong-Jun Lim2
, Jeonghoon Ha2, Kwangsoon Kim3, Ja Seong Bae3, Jeong Soo Kim3, Sungju Kim4, Dong-Jun Lim2 , Chul-Min Kim5
, Chul-Min Kim5
-
Endocrinology and Metabolism 2024;39(2):310-323.
DOI: https://doi.org/10.3803/EnM.2023.1870
Published online: April 9, 2024
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
3Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
4Healthcare Group, Lee & Ko, Seoul, Korea
5Department of Family Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding authors: Dong-Jun Lim. Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-6009, Fax: +82-2-599-3589, E-mail: ldj6026@catholic.ac.kr
- Chul-Min Kim. Department of Family Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-1760, Fax: +82-2-599-3589, E-mail: musofm@catholic.ac.kr
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,042 Views
- 14 Download
ABSTRACT
-
Background
- There is debate about ultrasonography screening for thyroid cancer and its cost-effectiveness. This study aimed to evaluate the cost-effectiveness of early screening (ES) versus symptomatic detection (SD) for differentiated thyroid cancer (DTC) in Korea.
-
Methods
- A Markov decision analysis model was constructed to compare the cost-effectiveness of ES and SD. The model considered direct medical costs, health outcomes, and different diagnostic and treatment pathways. Input data were derived from literature and Korean population studies. Incremental cost-effectiveness ratio (ICER) was calculated. Willingness-to-pay (WTP) threshold was set at USD 100,000 or 20,000 per quality-adjusted life year (QALY) gained. Sensitivity analyses were conducted to address uncertainties of the model’s variables.
-
Results
- In a base case scenario with 50 years of follow-up, ES was found to be cost-effective compared to SD, with an ICER of $2,852 per QALY. With WTP set at $100,000, in the case with follow-up less than 10 years, the SD was cost-effective. Sensitivity analysis showed that variables such as lobectomy probability, age, mortality, and utility scores significantly influenced the ICER. Despite variations in costs and other factors, all ICER values remained below the WTP threshold.
-
Conclusion
- Findings of this study indicate that ES is a cost-effective strategy for DTC screening in the Korean medical system. Early detection and subsequent lobectomy contribute to the cost-effectiveness of ES, while SD at an advanced stage makes ES more cost-effective. Expected follow-up duration should be considered to determine an optimal strategy for DTC screening.
- Thyroid cancer is one of the most common cancers in South Korea. Its incidence rate has been increasing in recent years [1-3]. Differentiated thyroid cancer (DTC) accounts for the majority of thyroid cancer cases in Korea [4]. Ultrasonography (US) is mostly used for thyroid cancer detection because it is safe and easily accessible. In addition, US allows for early detection of thyroid cancer [5]. However, there has been debate regarding the role of US in early screening (ES) of asymptomatic patients [6]. When thyroid US was performed on asymptomatic patients, the incidence of papillary thyroid cancer increased rapidly, but thyroid cancer mortality did not increase, raising the issue of ‘over-diagnosis’ [7]. Afterwards, a nationwide Korean study pointed out that the rapid increase in thyroid cancer was the result of over detection of small tumors, and efforts were made to reduce ‘unnecessary thyroid US examination’ [8].
- Meanwhile, there is a report showing that re-increase in thyroid cancer incidence, despite that the number of fine needle aspiration is not increased in the same period, indicating that there could be other reasons other than US screening [1]. In addition, recently, a few studies have shown that ES of DTC through US and treatment of DTC are crucial for improving patient outcomes including mortality, emphasizing the benefit of screening thyroid cancer [6,9]. In a study using nationwide data of about 4,000 people, the clinical suspicion group, that was not incidentally detected, showed 1.4 times higher all-cause mortality and about three times higher thyroid cancer-specific mortality than the asymptomatic group [9]. In one meta-analysis, incidentally detected thyroid cancer group had smaller tumors and lower incidence of aggressive histology, lymph node metastasis, or distant metastasis [6].
- In this regards, according to the Korean Thyroid Association guidelines, if there are three or more patients with DTC among immediate family members, screening tests performed on family members can lead to early diagnosis, but there is no evidence that it reduces morbidity and mortality, so US for this purpose is not recommended [10]. According to Korean national cancer screening guideline, thyroid cancer screening using US in asymptomatic adults is not recommended as a routine screening test because there is insufficient medical and scientific evidence to recommend it. However, if the patient wish to be screened for thyroid cancer, the test can be performed after providing appropriate information about the benefits and risks of screening [11].
- However, apart from medical outcome of cancer screening, there is controversy about the economic evaluation of cancer screening tools. For thyroid cancer, Yang et al. [12] have conducted a cost-effectiveness analysis to compare US screening and non-screening. The incremental cost-effectiveness ratio (ICER) in their research was $106,948/quality-adjusted life year (QALY). The authors concluded that US screening was not cost-effective [12]. However, in their study, the stage or treatment strategy of thyroid cancer was simplified to just one situation, rather than for each different situation (e.g., early or advanced state, lobectomy or total thyroidectomy [TT]).
- In addition, due to indolent characteristics of thyroid cancers, they have to be monitored for a longer time, similar to chronic diseases such as diabetes and hypertension [13,14]. As a result, thyroid cancer patients generally have heavier financial burdens and higher bankruptcy than other cancer survivals [15]. Therefore, whether it is possible to reduce costs by performing ES with US to reduce the extent of disease progression and treatment complications is questionable. In the study of Cham et al. [16], non-selective US for obese people without any risk factors (family history of thyroid cancer, history of significant radiation exposure, Hashimoto’s thyroiditis, and/or elevated thyrotropin) for thyroid cancer was not cost-effective. The authors indicated that thyroid cancer treatment and follow-up are long and extensive. Thus, US screening might cause over-diagnosis and the requirement of surveillance. However, the cost of US was based on cost in USA known to be higher than that in Korea.
- Therefore, the purpose of this study was to evaluate the cost-effectiveness of ES versus symptomatic detection (SD) for DTC in Korea. For this purpose, we built a cost-effectiveness analysis model using the Markov model and conducted a comprehensive cost-effectiveness analysis, considering both direct medical costs and potential health outcomes associated with each screening method.
INTRODUCTION
- Base case scenario and cost-utility analysis model construction
- This study created a Markov state-transition decision analysis model to analyze the cost-utility of ES for thyroid cancer. We compared the following two strategies: ES and SD. This diagnostic process was taken from previous literature. Moon et al. [9] have provided data from the National Epidemiologic Survey of Thyroid cancer (NEST) study, a retrospective nationwide study in Korea [17]. Through medical record review, cancer detection route was divided into a screening group and a clinical suspicion group. The ES group was diagnosed during cancer screening or incidentally diagnosed during treatment for diseases other than the thyroid. The clinical suspicion (SD) group was diagnosed from an examination for symptoms related to thyroid disease, such as throat pain and palpable mass.
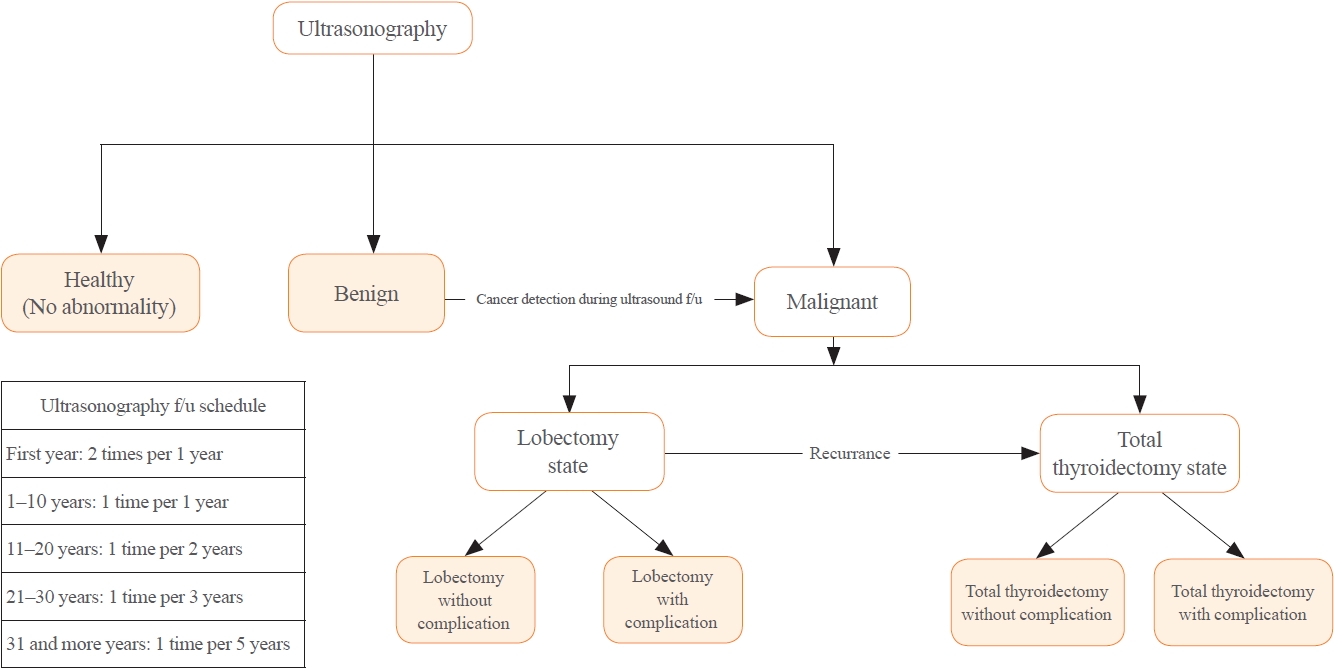
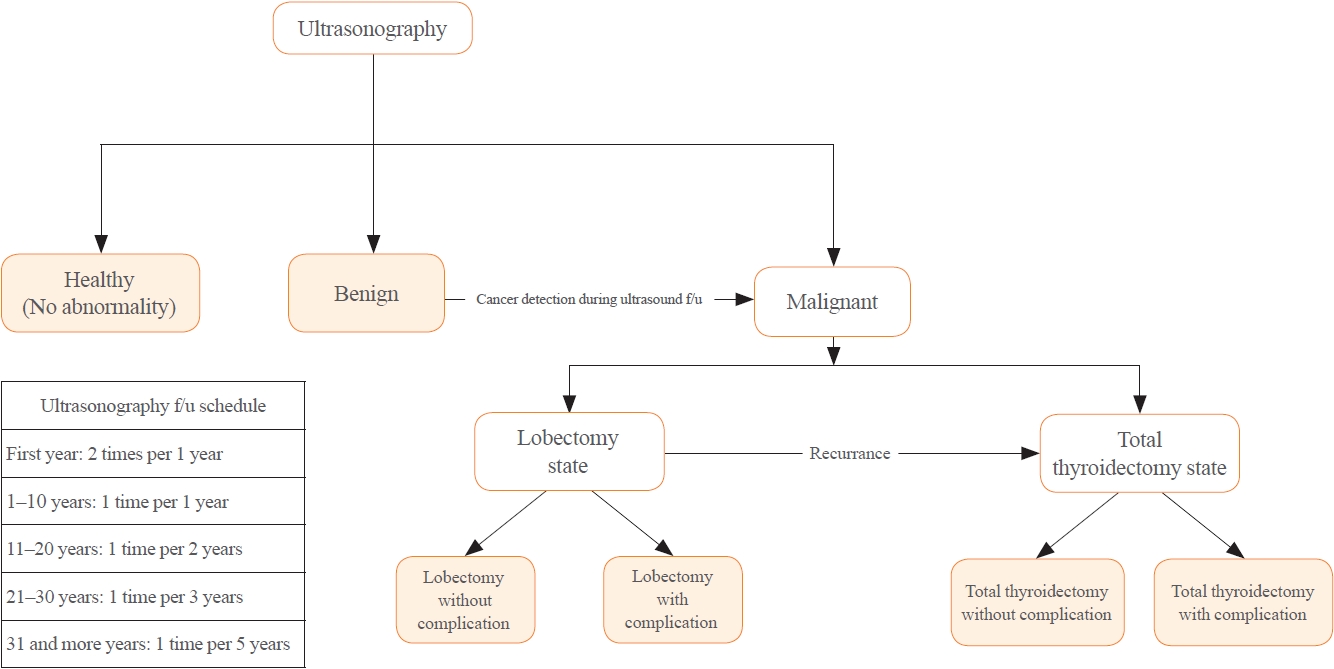
- Although the tracing process for thyroid nodules with ES or SD route is the same, cancer detection rate, cancer stage, recurrence rate, and mortality of ES and SD cohorts in previous literature are different [6,9,18]. Thus, the analysis model was built by reflecting these differences. In the base case scenario with the ES route, all healthy 40-year-old subject underwent thyroid US. On the other hand, with the SD route, selected subjects with symptoms related to thyroid disease underwent thyroid US. There might be no nodules in US results. A benign nodule or a malignancy might also be diagnosed. When a benign nodule is diagnosed, an US follow-up test is performed according to the schedule suggested in the previous literature [19]. If malignancy is diagnosed during US tracking or for the first time, lobectomy or TT and radioactive iodine (RAI) treatment are given according to clinical guidelines and cancer staging [20,21]. After treatment for malignancy, a follow-up US is performed for detection of recurrence. If cancer recurrence is detected, revision thyroidectomy or repeated radioiodine therapy or tyrosine kinase inhibitor (TKI) treatment could be used. In all processes, death due to thyroid disease or others was considered. The clinical scenario is summarized in Fig. 1.
- To reflect this scenario, the following seven health states were assumed in the analysis model: healthy, benign, post-hemithyroidectomy surveillance without complication, post-hemithyroidectomy surveillance with complication, post-TT surveillance without complication, post-TT surveillance with complication, and death (Supplemental Figs. S1, S2A) [22,23]. We considered long-term complications such as hypocalcemia induced by post-procedural hypoparathyroidism or hypothyroidism by mostly thyroidectomy in defining the health status. Transient complications such as recurrent laryngeal nerve injury and transient hypocalcemia were considered as transient health status in the analysis model.
- The model was cycled annually. As we assumed a 40-yearold patient as the base case, considering the peak-incidence age of thyroid cancer in Korea [2,24], termination of follow-up year ranged from 1 year to 50 years. As the current life expectancy of Koreans is about 83 years [25], a 40-year-old patient was assumed to survive until the age of 90, and a period of 50 years was assumed. Age-based probability for mortality was calculated based on both disease-specific mortality in a Korean cohort population [9,26] and a recently published meta-analysis study [6]. Age-dependent all-cause mortality was obtained from the “life table” produced by the Korean National Statistical Office [27].
- Cost and QALYs were tracked and discounted at a rate of 3% or 4.5% according to a previous study and the current Health Insurance Review & Assessment Service (HIRA) guidelines [28,29]. Willingness-to-pay (WTP) was set at US dollar (USD) 100,000 or 20,000, based on a previous study and current HIRA guidelines [28,30-32]. In the sensitivity analysis, the WTP was set at $20,000. Since there is no clear threshold for WTP in Korea, sensitivity analysis including cost-effectiveness acceptability curve analysis was also conducted [32,33]. TreeAge Pro software (TreeAge, Williamstown, MA, USA) was used to construct and analyze the model. Incremental cost (IC), incremental effectiveness (IE), ICER, and net monetary benefit (NMB) were calculated as follows:
- Input data
- The input data used in the model are summarized in Table 1 [6,9,12,18,24,26,29,34-43]. Only direct medical costs were considered for cost estimation. Direct costs included physician visiting fee, blood test fee, procedure costs for US, biopsy, surgery or RAI therapy, cost for TKI treatment, and costs of drugs, including levothyroxine or calcium/vitamin D. We did not consider indirect costs such as transportation costs or productivity loss. These costs were based on the 2023 HIRA publication [34]. In this regard, our study has a healthcare payer cost perspective [44].
- The schedule of US follow-up was based on a scenario from a previous study [29]. The ultrasound follow-up schedule was once every 6 months for the first year, annual follow-up from 2 to 5 years, every 2 years for 5 to 10 years, every 3 years for 10 to 20 years, and every 5 years after 20 years. All costs were estimated in Korean Republic won (KRW) and converted into USD by applying an exchange rate of 1 USD to 1,300 KRW (May 1, 2023), before being inserted into the model. In Korea, medical expenses are set differently depending on whether it is a primary care clinic or a tertiary hospital. Usually, a tertiary medical institution has a higher cost than a primary medical institution. Base costs were estimated according to costs of a tertiary hospital.
- Transition probabilities were calculated based on previous literature, especially studies with Korean populations [18,35-38]. According to previous literature, different transition probabilities were substituted according to the ES or SD strategy [9,39]. Especially, transient probabilities of benign thyroid nodules were brought from a previous study that conducted cost-effectiveness analysis of thyroid US for thyroid cancer screening [12]. This previous study also calculated transient probabilities based on the study from Korea [12]. In addition, the cancer detection rate was taken from the prevalence of thyroid nodules and thyroid cancer in healthy subjects by US [18]. As cancer detection rates were different according to age [18,40], we reflected this difference. According to a previous study, cancer stage is different depending on ES or SD and the possibility of lobectomy is also different accordingly [9]. Differences in all-cause mortality and thyroid-specific mortality according to ES and SD were also reflected [6,35,36]. Results and data in the above-mentioned literature were converted into transition probabilities using the formula suggested in a previous study [45]. Sensitivity analysis ranged from 50% to 150%.
- The utility score is a measure of the overall quality of life and patient preferences related to a specific health condition or treatment. Utility scores range from 0 to 1, with 1 indicating perfect health and 0 indicating death. Due to the lack of research on utility score of thyroid cancer in a Korean population, we used utility scores from studies involving various ethnics [41]. As it was also difficult to find the utility score of patients with benign thyroid nodules, the utility score from a previous cost-effectiveness analysis study was used [12]. Because there was no absolute utility score fit to our health status assumption, the minimum and maximum scores for each health status were used for sensitivity analysis.
- Statements for health economic analysis plan and approach to engagement with patients and others
- We asscess our research based on the assessement guideline, ‘Consolidated Health Economic Evaluation Reporting Standards’ [46]. The health economic analysis was planned from the researchers from the tertiary hospital. As it is a retrospective study, it was impossible to engage patients or service recipients.
- Ethical statement
- The research protocal was submitted to the Institutional Review Board (IRB) of Uijeongbu St. Mary’s Hospital, and it was confirmed that this study was exempt from IRB review, as this study utilized only publicly avaiable dataset and previosly published literature (UC23ZASI0129).
METHODS
- Base case analysis
- In the base case with a discount rate of 3%, the ES strategy incurred a cost of $9,208 and demonstrated an effectiveness of 23.26 QALYs, resulting in a NMB of $2,317,191 at WTP of $100,000 and $456,072 at WTP of $20,000. The SD strategy had a cost of $8,793 and achieved an effectiveness of 23.12 QALYs, resulting in NMB of $2,303,060 at WTP of $100,000 and $453,572 at WTP of $20,000. As the IC was $145 and the IE was 0.15, the calculated ICER for ES versus SD was $2,852 per QALY. In the scenario with a discount rate of 4.5%, the ES strategy had a cost of $6,518 with an IC of $371. It achieved an effectiveness of 18.52 QALYs with an IE of 0.09 QALYs. The ICER for the ES strategy at a discount rate of 4.5% was $4,226. The NMB of ES was calculated as $1,845,617 at WTP of $100,000. The SD strategy at a discount rate of 4.5% had a cost of $6,147 with an effectiveness of 18.43 QALYs, resulting in an NMB of $1,836,741 (Table 2).
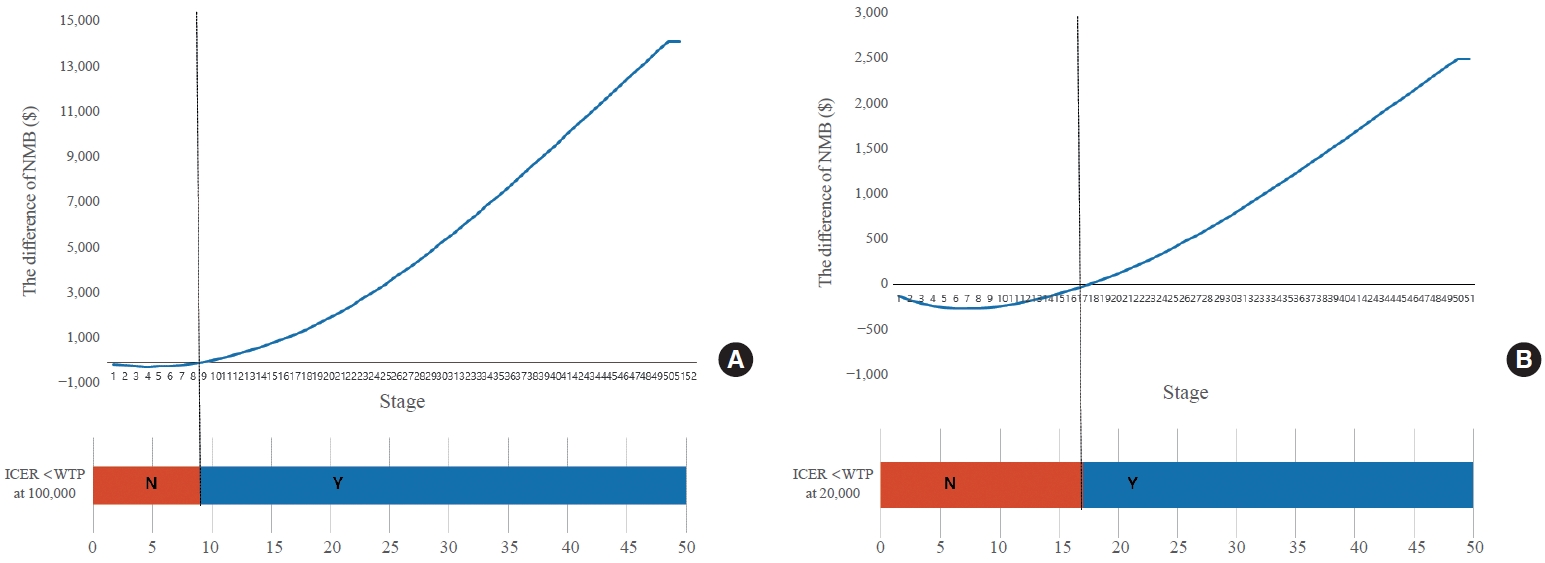
- ICER and NMB were observed according to the termination stage of the constructed analysis model. In the early stage, the NMB of ES was lower than the that of SD, resulting in a negative value of the difference in NMB between the two strategies. With WTP of $100,000, the difference of the NMB changed to a positive number at stage 10, meaning that the NMB of ES was higher than that of SD. Below stage 10, ICER exceeded $100,000. Thus, the ES was not cost-effective. At stage 10 and above, WTP was less than $100,000, making ES cost-effective. With WTP of $20,000, the difference of the NMB changed to positive number at stage 18, meaning that the NMB of ES was higher than that of SD. Below stage 18, WTP exceeded $20,000. Thus, the ES was not cost-effective. At stage 18 and above, WTP was less than $20,000, making ES cost-effective (Fig. 2, Supplemental Table S1).
- The results of Markov probability analysis for each strategy are described in Supplemental Fig. S2B, C. The cost-effectiveness acceptability curve analysis shows that the cost-effectiveness of two strategies crosses over at WTP around $5,000 (Supplemental Fig. S2D)
- Sensitivity analysis
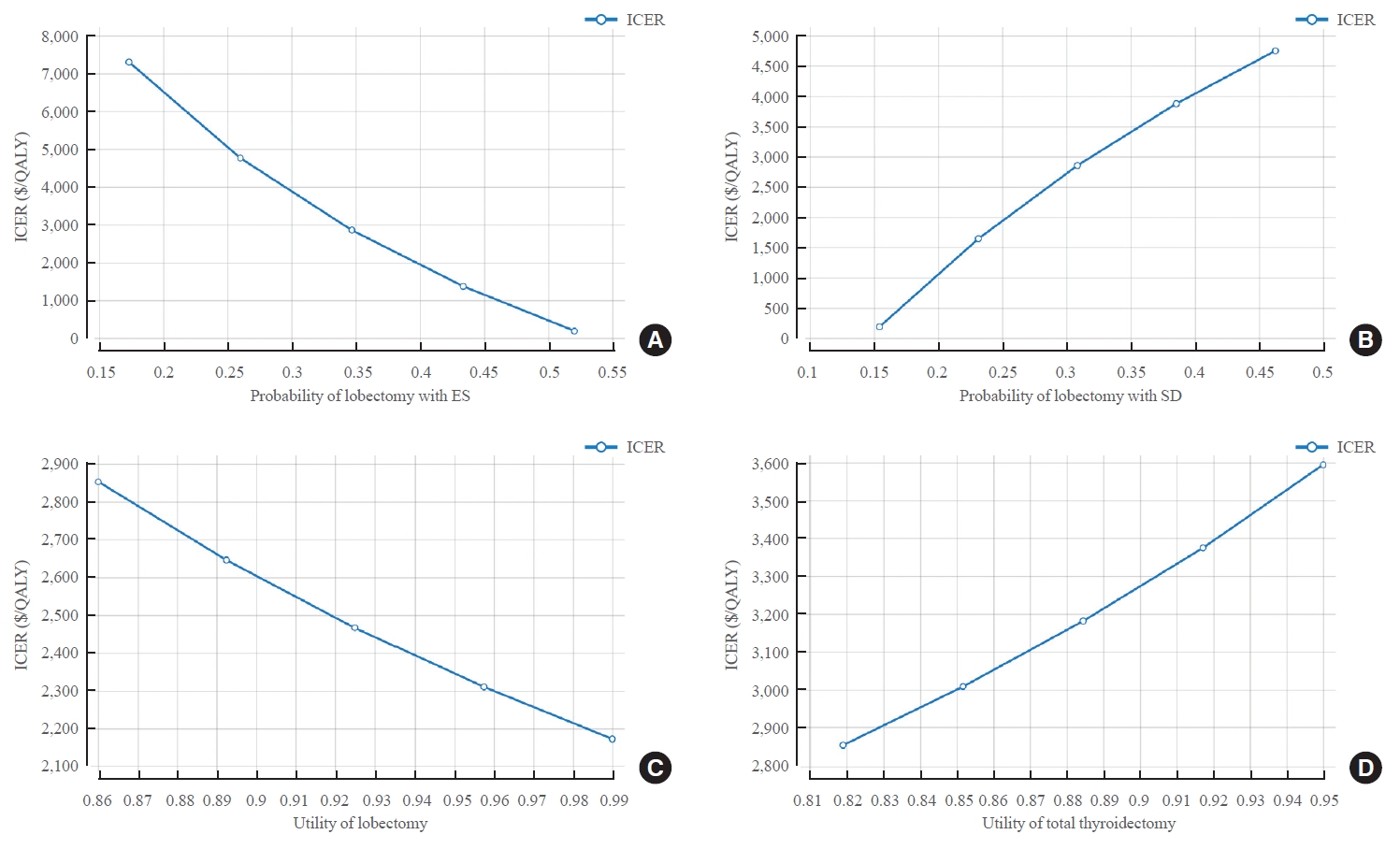
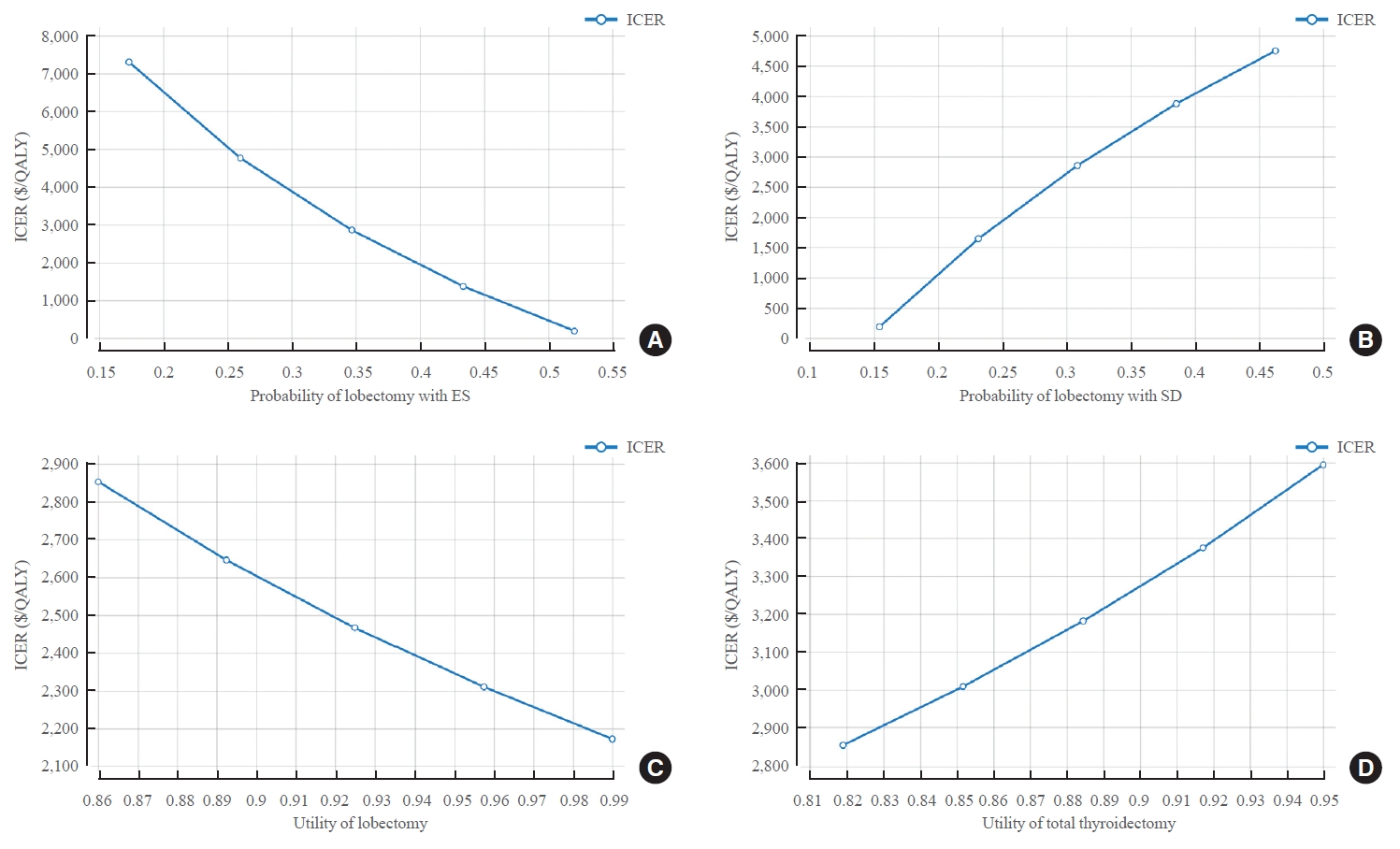
- With the ES strategy, the higher the probability of lobectomy value, the lower the ICER value (at probability of 0.1735, ICER=$7,299/QALY; at probability of 0.5205, ICER=$182/QALY). On the other hand, with the SD strategy, ICER increased as the probability of lobectomy increased (at probability of 0.1543, ICER=$187/QALY; at probability of 0.3086, ICER=$2,852/ QALY). Utility score also influenced model analysis. The higher the utility score after lobectomy, the more cost-effective ES was (ICER=$2,852/QALY when utility score after lobectomy was 0.86; and ICER=$2,172/QALY when utility score was 0.99). On the other hand, in the case of TT, the lower the utility score, the more cost-effective ES was (ICER=$2,852/QALY when the utility score after TT was 0.819; and ICER=$3,593/QALY when the utility score was 0.95) (Fig. 3, Supplemental Tables S2, S3).
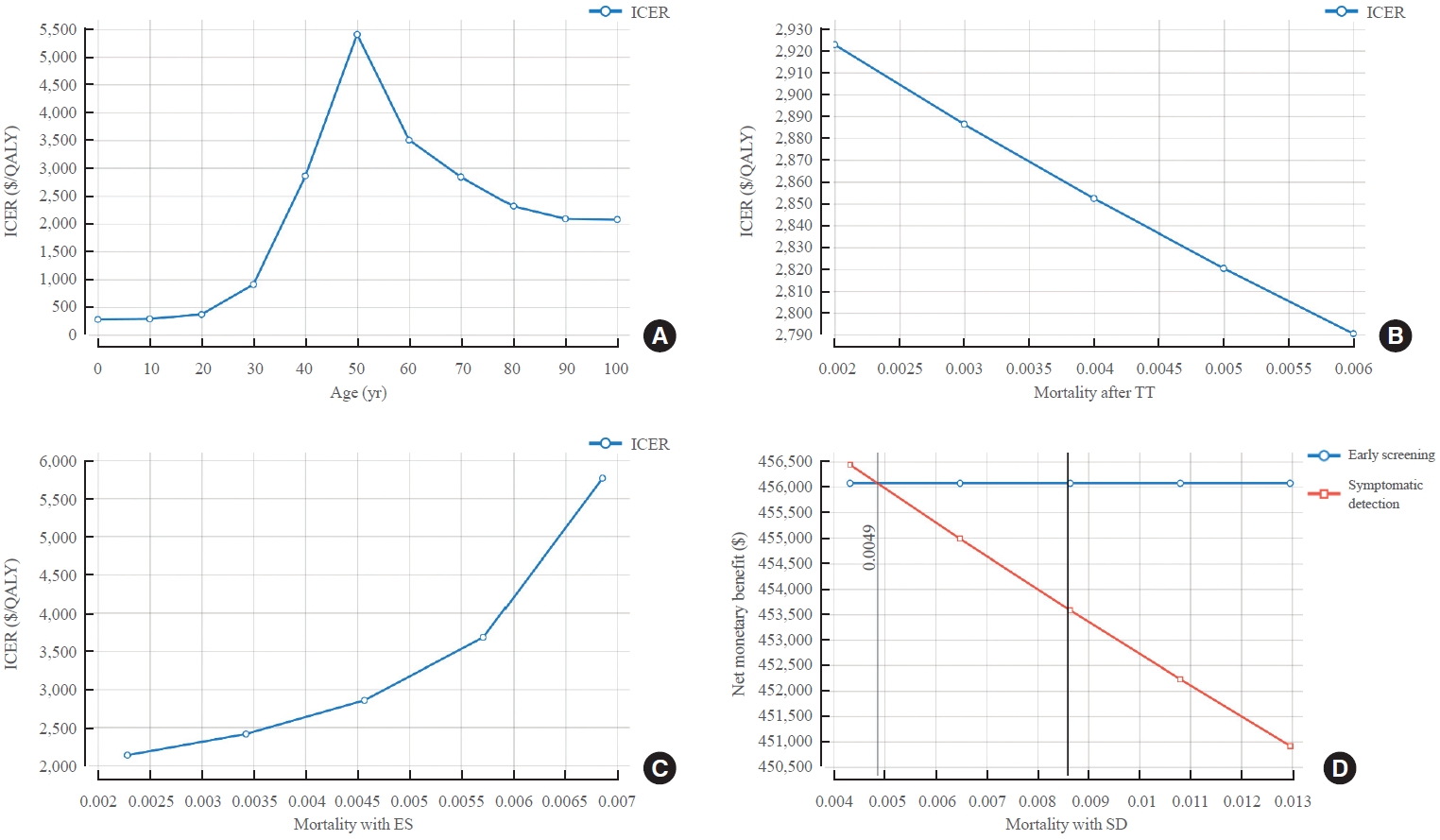
- Changes in ICER according to the age at US and mortality were also analyzed. ICER values increased from the 20s to the 50s and then decreased (ICER=$364/QALY at age of 20; ICER=$2,852/QALY at age of 40; ICER=$3,501/QALY at age of 60; and ICER=$2,314/QALY at age of 80). However, all ICER values were lower than WTP, which meant that ES was cost-effective. In addition, the higher the mortality after TT or the higher the mortality with the SD strategy, the lower the ICER value, meaning that the ES was more cost-effective (ICER=$2,923/QALY when mortality after TT was 0.002; ICER=$2,790/QALY when mortality after TT was 0.006; ICER=–$51,590/QALY when the mortality with the SD strategy was 0.0043; and ICER=$1,952/ QALY when the mortality was 0.0130). As mortality in SD strategy increased, the cost of SD strategy decreased while IC increased. On the other hand, the effect that could be obtained from SD strategy decreased and IE increased, which reduced ICER. With the ES strategy, the lower the mortality, the lower the ICER value. Thus, the ES was more cost-effective (ICER= $2,134/QALY when the mortality with ES was 0.0023; ICER= $5,759/QALY when mortality was 0.0069) (Fig. 4, Supplemental Table S4).
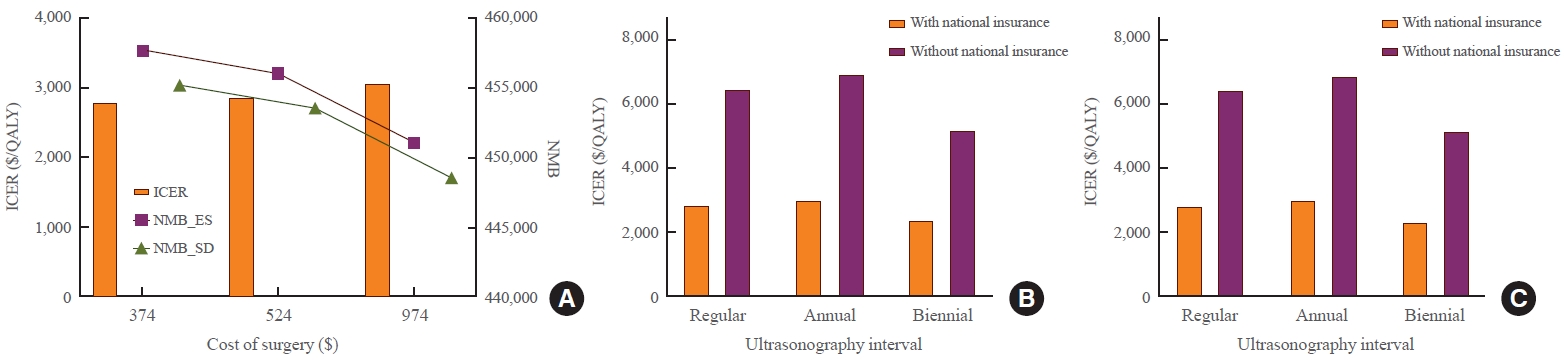
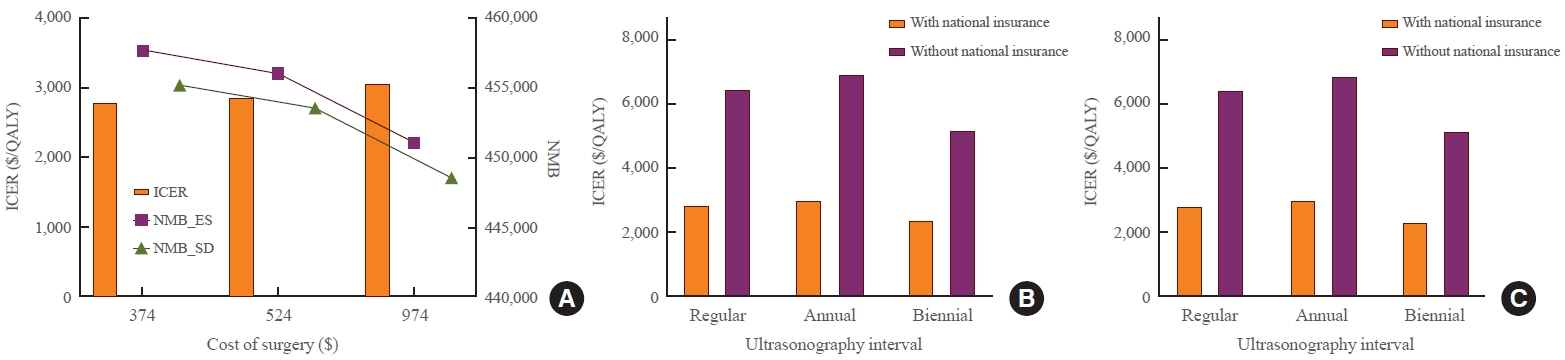
- To reflect the real clinical field, sensitivity analysis was performed while changing the lobectomy cost and thyroid US cost. As the cost of surgery and US increased, the NMB of ES and SD strategies decreased while the ICER increased. When the lobectomy cost was $374, the ICER was $2,783/QALY and the NMB was $457,742 for ES and $455,238 for SD. When the lobectomy cost was $974, the ICER was $3,059/QALY and the NMB was $451,059 for ES and $448,595 for SD. When US cost was based on a tertiary hospital, ICER was $2,852/QALY and NMB was $456,072 for ES and $453,577 for SD. When US cost was based on a primary care clinic, ICER was $2,350/QALY and NMB was $456,270 for ES and $453,702 for SD. The US tracing interval was also changed. In addition to the strategy of increasing the tracking interval over time in the base scenario, annual or biennial follow-up strategies were analyzed. With annual and biennial US follow-up, ICERs were $3,021/QALY and $2,359/QALY, respectively. Despite variations, all ICERs were under the WTP. In particular, even when it was assumed that national health insurance was not applied to the cost of US, the ICER was $6,470/QALY, which was lower than the WTP. As this cost, without national health insurance, included patient’s out-of-pocket cost, this sensitivity analysis included patient’s cost prospective. Without national insurance, the ICER was $6,904/QALY when annual follow-up was performed and $5,204/QALY when biennial follow-up was performed (Fig. 5, Supplemental Table S5).
RESULTS
- The base case analysis in our study showed that the ES strategy was more cost-effective than the SD strategy for thyroid cancer management. The ES incurred a slightly higher cost but achieved greater effectiveness, resulting in a higher NMB than the SD strategy. The ICER was below the WTP. It confirmed the cost-effectiveness of the ES strategy. Sensitivity analysis revealed that variables such as lobectomy probability, age, mortality, and utility scores significantly impacted the ICER. Despite variations in costs and other factors, all ICER values remained below the WTP threshold, reaffirming the cost-effectiveness of the ES strategy.
- However, Yang et al. [12] have shown that US screening for thyroid cancer has no advantage in terms of cost-effectiveness compared to non-screening, different from our results. In their model, US screening strategy incurred $18,819 with 18.74 QALY and the non-screening strategy incurred $15,864 with 18.71 QALY, showing that US screening had a higher cost with relatively small benefit. However, in their study, the stage or treatment strategy of thyroid cancer was not considered (e.g., early or advanced stage and lobectomy or TT). The authors simplified the complicated thyroid cancer management scenario into just one ‘treatment’ strategy. On the other hand, although subjects were limited to obese patients, one study showed that US screening was cost-effective for obese people with risk factors including family history of thyroid cancer, history of significant radiation exposure, Hashimoto’s thyroiditis, and/or elevated thyrotropin [16]. In the decision model constructed by the authors, the treatment strategy was different for each thyroid cancer stage and a cost-effectiveness analysis was performed accordingly [16]. Considering that treatment strategy is different according to thyroid cancer stage, it might influence the result of cost-effectiveness of US screening.
- The important influential factor in the created model for sensitivity analysis of our study was the probability of lobectomy. The probability of lobectomy is related to the stage of cancer [9,12,20,21]. The earlier the stage of cancer, the higher the probability of lobectomy, with decreased frequency of TT. With the ES strategy, a higher probability of lobectomy led to lower ICER values, indicating greater cost-effectiveness. Conversely, with the SD strategy, an increased probability of lobectomy resulted in higher ICER values, suggesting reduced cost-effectiveness. Thus, early detection of cancer and subsequent lobectomy can increase the cost-effectiveness of the ES strategy. In addition, SD at an advanced stage makes ES more cost-effective.
- Utility score also influenced model analysis. This is also associated with cancer stage. The higher the utility score after lobectomy, the more cost-effective the ES. This suggests that ES can be cost-effective if the utility score after lobectomy is high since thyroid cancer is likely to be detected by ES at an early stage and treated only with lobectomy, without increased number of postoperative complications. On the other hand, in the case of TT, the lower the utility score, the more cost-effective the ES. This means that there are more TT cases with the SD strategy, which has a greater impact. If the utility score was high without postoperative complications even after TT, the effectiveness obtained with SD could have been relatively high.
- In the study of Yang et al. [12], the cost of benign thyroid nodule follow-up had a great impact on the analysis. In our study, the ‘time’ was also an important factor. ICER values varied throughout stages of the analysis model (indicating follow-up duration). With WTP at $100,000, ES was not cost-effective before 10 years. However, it was cost-effective after 10 years. Therefore, if patients were planned to be followed up for a longer period, early US could be beneficial. In a cost-effectiveness study on US follow-up of thyroid nodules incidentally detected with computed tomography scan, US follow-up was more cost-effective the longer patients were followed and the patients were younger than 60, while for those over 60 years of age, the US follow-up was less cost-effective [47]. Comparing this study with our study, early detection and early treatment can be economically advantageous, because despite as the follow-up period becomes longer, the cumulative cost of ultrasound increases, but the cumulative benefit obtained from the US follow-up also increased. Regarding age, performing thyroid evaluation starting at age 50 had a peak ICER value. It might be because the probability of malignancy detection was higher in a population under the age 50 years old. Thus, younger patients could have more benefit with early detection of malignancy as the detection rate was relatively high. In addition, they could be followed up for a longer time. On the other hand, for older adults, as all-cause mortality increased, the cost spent decreased, resulting in ICER decrease.
- Therefore, higher mortality rates after TT, or mortality in the SD strategy led to lower ICER values. Meanwhile, higher mortality rates with the ES strategy led to higher ICER values. It meant that ES strategy was relatively beneficial for effectiveness. Although the cost could be lower as mortality increased, resulting in a short follow-up duration, the obtained effectiveness was also decreased, resulting in ICER increase. In summary, it is better to screen early and manage thyroid nodule quickly when the patient is young, only from the standpoint view of the cost-utility. On the other hand, if the expected lifetime is short and the follow-up period is expected to be short, there is no need to do ES.
- Even if the ‘time’ was corrected, the cost might have been underestimated in our study. In the study of Yang et al. [12], total cost was calculated by summing up direct medical costs and productivity loss considering hospitalization and labor loss. However, such indirect costs are difficult to measure. They might introduce uncertainties [48]. In addition to the indirect cost issue, the medical cost is relatively low in Korea [13]. To improve this issue, we conducted sensitivity analysis while varying costs of surgery and US. In other words, the total cost may be underestimated because indirect cost is not considered. However, by analyzing a wide range of costs, the results when indirect cost is included were also estimated; In another previous study, there was a study that calculated the indirect cost incurred during surgery to be about $481 [29], and we attempted to set an upper limit for surgical costs including this cost. Even when we increased the cost of surgery up to two times based on the cost of scenario, the ES was still cost-effective. In particular, ES was cost-effective even when it was assumed that the cost of US could not be covered by national insurance and the cost went up. After changing the US follow-up interval, all ICER values according to follow-up interval were lower than the WTP. This suggests that the US interval in current practice is reasonable.
- Our study has some limitations. First, transient probabilities used in our analysis were mostly obtained from retrospective studies. As such studies mostly focused on surgical results, post-treatment complications might have been underestimated. In addition, most studies used as references might have biases because these studies were based on hospital data. Although some studies were based on national data, these studies targeted patients diagnosed with thyroid cancer. Thus, not all populations were targets of our study. In particular, as the average age of patients diagnosed with thyroid cancer was over 40 years in previous study based on the Korean national population, the clinical pathway of younger patients was not fully reflected in our analysis. Although we changed the transient probabilities according to different detection rates in each age group, in order to obtain accurate medical and economic outcomes in actual young patients, a prospective study including younger patients is considered necessary.
- Second, although the utility score used in this study were obtained from a systematic review on utility scores of thyroid cancer patients, it was hard to say that it completely fit our health status assumption. This was because the cohorts of each study were different from ours and most studies that produced utility scores were retrospective. Therefore, it is difficult to reveal the reliability and validity of utility scores, and the utility scores do not accurately reflect the Korean health care system. However, to overcome this limitation, we performed sensitivity analysis by changing the utility score. In addition, as this is a retrospective study, there is a limitation in that all study results required for the thyroid nodule tracking model in Korea are not available; Most studies are the results of research conducted when cancer has already been diagnosed. Some values were used directly from overseas literature research results and may not fit the Korean situation at all. However, we tried to borrow the results derived from the Korean study as much as possible. In addition, as we also considered the detection of benign nodules and follow-up, our study reflects the real world practice.
- Finally, we did not consider indeterminate cytology results such as the follicular neoplasm, atypia of unknown significance, or active surveillance of micro-papillary thyroid carcinoma in nodules due to a lack of data and for simplification of analysis. Thus, in the future, efforts should be made to obtain a utility score for each clinical situation of thyroid cancer in Korea, systematic data collection on thyroid nodules, and biopsy results. Prospective follow-up studies are also needed. Despite these limitations, this study reveals that the ES strategy is cost-effective in diagnosing thyroid cancer. Based on our study results, although it can be argued that all populations should be screened for thyroid cancer, at least it shows that there may be no need to prevent or ban screening of thyroid cancer.
- Overall, the ES strategy was found to be more cost-effective than the SD strategy in diagnosing thyroid cancer in Korean medical system. In particular, early detection of cancer and subsequent lobectomy can increase the cost-effectiveness of the ES strategy. On the other hands, if age and mortality rate are high and the follow-up period is expected to be short, ES is less cost-effective. This study provides valuable insights for policymakers and healthcare providers regarding optimal DTC screening strategies in Korea.
DISCUSSION
Supplementary Material
Supplemental Table S1.
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
Supplemental Table S5.
Supplemental Fig. S1.
Supplemental Fig. S2.
-
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest concerning the publication of this paper. Author Sungju Kim is affiliated with Healthcare Group, Lee & Ko., a legal entity. This affiliation does not pose any financial conflict of interest in relation to the research presented in this manuscript.
-
AUTHOR CONTRIBUTIONS
Conception or design: H.S.B. Acquisition, analysis, or interpretation of data: H.S.B., J.H., K.K., J.S.B., J.S.K., S.K., D.J.L., C.M.K. Drafting the work or revising: H.S.B., S.K., D.J.L., C.M.K. Final approval of the manuscript: H.S.B., J.H., K.K., J.S.B., J.S.K., S.K., D.J.L., C.M.K.
Article information
-
Acknowledgements
- This research was supported by a grant (grant numbers: HC19C 0215) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea.
- We would like to extend our sincere gratitude to our research nurse, Jeongeun Lee, for her invaluable assistance and dedication to our study, especially in the collection and organization of data.

| Variable | Base case value | Analysis range | Reference |
|---|---|---|---|
| Cost, USD (KRW) | |||
| Cost of physician visit | 19 (24,760) | 10–23 (12,380–30,180) | [34] |
| Cost of blood test | 92 (120,100) | 72–92 (94,220–120,100) | [34] |
| Cost of T4 replacement | 10 (13,110) | 8–26 (11,650–33,185) | [34] |
| Cost of calcium replacement | 52 (67,495) | 8–52 (10,950–67,495) | [34] |
| Cost of FNA | 79 (103,140) | 64–79 (82,910–103,140) | [34] |
| Cost of RAI | 283 (367,900) | 218–283 (283,400–367,900) | [34] |
| Cost of surgery (lobectomy) | 524 (681,210) | 374–742 (486,490–965,050) | [29,34] |
| Cost for transient vocal cord injury | 31 (40,300) | 18–31 (23,400–40,300) | [29] |
| Cost of ultrasonography at tertiary hospital | 60 (77,990) | [34] | |
| Cost of ultrasonography at primary hospital | 47 (61,360) | [34] | |
| Cost of ultrasonography without national insurance | 154 (200,000) | Cost in the reference hospital | |
| Probability | |||
| Probability of lobectomy among ES population | 0.3470 | 0.1735–0.5205 | [9] |
| Probability of lobectomy among SD population | 0.3086 | 0.1543–0.4628 | [9] |
| Probability of rai-refractory thyroid cancer | 0.0087 | 0.0043–0.0131 | [42] |
| Probability of cancer during benign nodule follow-up | 0.033 | 0.0163–0.0487 | [18] |
| Probability of permanent hypocalcemia among ES population | 0.0045 | 0.0022–0.0067 | [39] |
| Probability of permanent hypocalcemia among SD population | 0.0072 | 0.0036–0.0108 | [39] |
| Probability of transient laryngeal nerve injury among ES population | 0.0004 | 0.0002–0.0006 | [39] |
| Probability of transient laryngeal nerve injury among SD population | 0.008 | 0.0040–0.0121 | [39] |
| Probability of complication after HT | 0.1941 | 0.0971–0.2912 | [38] |
| Probability of locoregional recurrence after HT | 0.0045 | 0.0023–0.0068 | [35-37] |
| Probability of complication after TT | 0.0217 | 0.0109–0.0326 | [35-37] |
| Probability of locoregional recurrence after TT | 0.0019 | 0.0010–0.0029 | [35-37] |
| Mortality | [24,26] | ||
| Probability of death after HT | 0.0039 | 0.0020–0.0059 | [24,26] |
| Probability of death after TT | 0.0040 | 0.0020–0.0060 | [24,26] |
| Probability of death among TKI treated patient | 0.0038 | 0.0019–0.0057 | [43] |
| Probability of death among ES population | 0.0046 | 0.0023–0.0069 | [6,9] |
| Probability of death among SD population | 0.0087 | 0.0043–0.0130 | [6,9] |
| Time dependent probability | |||
| Probability of benign nodule detection | |||
| Age <40 | 0.119 | 0.0598–0.1795 | [18] |
| Age of 40–49 | 0.1675 | 0.0838–0.2513 | [18] |
| Age of 50–59 | 0.1894 | 0.0947–0.2842 | [18] |
| Age ≥60 | 0.3079 | 0.1540–0.4619 | [18] |
| Probability of cancer detection with screening | |||
| Age <40 | 0.0112 | 0.0056–0.0167 | [18] |
| Age of 40–49 | 0.0115 | 0.0058–0.0173 | [18] |
| Age of 50–59 | 0.0131 | 0.0065–0.0196 | [18] |
| Age ≥60 | 0.0149 | 0.0074–0.0023 | [18] |
| Probability of cancer detection with symptom detection | |||
| Age <30 | 0.0154 | 0.0077–0.0231 | [40] |
| Age of 30–39 | 0.0146 | 0.0073–0.0219 | [40] |
| Age of 40–49 | 0.0116 | 0.0058–0.0174 | [40] |
| Age of 50–59 | 0.0087 | 0.0043–0.0130 | [40] |
| Age of 60–69 | 0.0087 | 0.0043–0.0130 | [40] |
| Age ≥70 | 0.0080 | 0.0040–0.0119 | [40] |
| Utility | |||
| Utility of continued patients with benign thyroid nodule | 0.99 | 0.89–1.00 | [12,41] |
| Utility of post-HT surveillance without complication | 0.86 | 0.86–0.99 | [41] |
| Utility of post-HT surveillance with complication | 0.82 | 0.82–0.875 | [41] |
| Utility of post-TT surveillance without complication | 0.819 | 0.819–0.95 | [41] |
| Utility of post-TT surveillance with complication | 0.778 | 0.778–0.88 | [41] |
| Utility of patients who have recurrence of cancer | 0.54 | 0.49–0.59 | [12,41] |
| Utility of patients who have laryngeal injury | 0.627 | 0.205–0.627 | [41] |
| Utility of TKI use | 0.72 | 0.72–0.883 | [41] |
- 1. Jung CK, Bae JS, Park YJ. Re-increasing trends in thyroid cancer incidence after a short period of decrease in Korea: reigniting the debate on ultrasound screening. Endocrinol Metab (Seoul) 2022;37:816–8.ArticlePubMedPMCPDF
- 2. Choi YM, Lee J, Kwak MK, Jeon MJ, Kim TY, Hong EG, et al. Recent changes in the incidence of thyroid cancer in Korea between 2005 and 2018: analysis of Korean National Data. Endocrinol Metab (Seoul) 2022;37:791–9.ArticlePubMedPMCPDF
- 3. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317–22.ArticlePubMed
- 4. Lee JY, Baek JH, Ha EJ, Sung JY, Shin JH, Kim JH, et al. 2020 Imaging guidelines for thyroid nodules and differentiated thyroid cancer: Korean Society of Thyroid Radiology. Korean J Radiol 2021;22:840–60.ArticlePubMedPMCPDF
- 5. Baek JH. Thyroid cancer screening: how to maximize its benefits and minimize its harms. Endocrinol Metab (Seoul) 2023;38:75–7.ArticlePubMedPMCPDF
- 6. Moon S, Song YS, Jung KY, Lee EK, Park YJ. Lower thyroid cancer mortality in patients detected by screening: a meta-analysis. Endocrinol Metab (Seoul) 2023;38:93–103.ArticlePubMedPMCPDF
- 7. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med 2014;371:1765–7.ArticlePubMed
- 8. Park S, Oh CM, Cho H, Lee JY, Jung KW, Jun JK, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ 2016;355:i5745.ArticlePubMedPMC
- 9. Moon S, Lee EK, Choi H, Park SK, Park YJ. Survival comparison of incidentally found versus clinically detected thyroid cancers: an analysis of a nationwide cohort study. Endocrinol Metab (Seoul) 2023;38:81–92.ArticlePubMedPMCPDF
- 10. Park YJ, Lee EK, Song YS, Kang SH, Koo BS, Kim SW, et al. 2023 Korean Thyroid Association management guidelines for patients with thyroid nodules. Int J Thyroidol 2023;16:1–31.Article
- 11. Yi KH, Kim SY, Kim DH, Kim SW, Na DG, Lee YJ, et al. The Korean guideline for thyroid cancer screening. J Korean Med Assoc 2015;58:302–12.
- 12. Yang N, Yang H, Guo JJ, Hu M, Li S. Cost-effectiveness analysis of ultrasound screening for thyroid cancer in asymptomatic adults. Front Public Health 2021;9:729684.ArticlePubMedPMC
- 13. Baek HS, Jeong CH, Ha J, Bae JS, Kim JS, Lim DJ, et al. Cost-effectiveness analysis of active surveillance compared to early surgery in small papillary thyroid cancer: a systemic review. Cancer Manag Res 2021;13:6721–30.ArticlePubMedPMCPDF
- 14. Kim K, Kim M, Lim W, Kim BH, Park SK. The concept of economic evaluation and its application in thyroid cancer research. Endocrinol Metab (Seoul) 2021;36:725–36.ArticlePubMedPMCPDF
- 15. Uppal N, Cunningham Nee Lubitz C, James B. The cost and financial burden of thyroid cancer on patients in the US: a review and directions for future research. JAMA Otolaryngol Head Neck Surg 2022;148:568–75.ArticlePubMed
- 16. Cham S, Zanocco K, Sturgeon C, Yeh MW, Harari A. Riskbased ultrasound screening for thyroid cancer in obese patients is cost-effective. Thyroid 2014;24:975–86.ArticlePubMedPMC
- 17. Oh CM, Kong HJ, Kim E, Kim H, Jung KW, Park S, et al. National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Epidemiol Health 2018;40:e2018052.ArticlePubMedPMC
- 18. Oh EY, Jang HW, Lee JI, Kim HK, Kim SW, Chung JH. Prevalence of thyroid nodules and cancer detected by ultrasonography in healthy Korean adults: clinical features and the risk for malignancy. Int J Thyroidol 2010;3:142–8.
- 19. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2021;22:2094–123.ArticlePubMedPMCPDF
- 20. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1856–83.ArticlePubMedPDF
- 21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133.PubMedPMC
- 22. Gupta N, Verma R, Dhiman RK, Rajsekhar K, Prinja S. Cost-effectiveness analysis and decision modelling: a tutorial for clinicians. J Clin Exp Hepatol 2020;10:177–84.ArticlePubMedPMC
- 23. Baek HS, Ha J, Kim K, Bae J, Kim JS, Kim S, et al. Cost-effectiveness of active surveillance compared to early surgery of small papillary thyroid cancer: a retrospective study on a Korean population. J Korean Med Sci 2023;38:e264.ArticlePubMedPMCPDF
- 24. National Cancer Information Center. Cancer statistics according to age [Internet]. Goyang: National Cancer Information Center; 2024 [cited 2024 Feb 4]. Available from: https://www.cancer.go.kr/lay1/S1T639C642/contents.do.
- 25. Statistics Korea. Life expectancy [Internet]. Daejeon: Statistics Korea; 2023 [cited 2024 Feb 4]. Available from: https://www.index.go.kr/unify/idx-info.do?idxCd=8016.
- 26. Zhang HS, Lee EK, Jung YS, Nam BH, Jung KW, Kong HJ, et al. Total thyroidectomy’s association with survival in papillary thyroid cancers and the high proportion of total thyroidectomy in low-risk patients: analysis of Korean nationwide data. Surgery 2019;165:629–36.ArticlePubMed
- 27. Korean Statistical Information Service. Life table [Internet]. Daejeon: Statistics Korea; 2023 [cited 2024 Feb 4]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B42&conn_path=I2.
- 28. Health Insurance Review & Assessment Service. 2021 Economic evaluation guidelines in medicine and drugs [Internet]. Wonju: HIRA; 2021 [cited 2024 Feb 4]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=8661.
- 29. Kim K, Choi JY, Kim SJ, Lee EK, Lee YK, Ryu JS, et al. Active surveillance versus immediate surgery for low-risk papillary thyroid microcarcinoma patients in South Korea: a cost-minimization analysis from the MAeSTro Study. Thyroid 2022;32:648–56.ArticlePubMed
- 30. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94:925–30.ArticlePubMedPMC
- 31. Kim YH, Shin SJ, Kim YJ, Lee HJ, Lee SK, Park SY, et al. NECA’s methodological guide for economic evaluation of medical intervention. Seoul: National Evidence-based Healthcare Collaborating Agency; 2021.
- 32. Health Insurance Review & Assessment Service. 2018-2022 ICER [Internet]. Wonju: 2023 [cited 2024 Feb 4]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=10648.
- 33. Health Insurance Review & Assessment Service. ICER first published 2022 [Internet]. Wonju: 2022 [cited 2024 Feb 4]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=10774&pageIndex=1&pageIndex2=1#none.
- 34. Health Insurance Review & Assessment Service. Medical fee schedule of health service in Korea under national health insurance coverage [Internet]. Wonju: 2021 [cited 2024 Feb 4]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=9105.
- 35. Kwon H, Jeon MJ, Kim WG, Park S, Kim M, Song DE, et al. A comparison of lobectomy and total thyroidectomy in patients with papillary thyroid microcarcinoma: a retrospective individual risk factor-matched cohort study. Eur J Endocrinol 2017;176:371–8.ArticlePubMed
- 36. Jeon YW, Gwak HG, Lim ST, Schneider J, Suh YJ. Longterm prognosis of unilateral and multifocal papillary thyroid microcarcinoma after unilateral lobectomy versus total thyroidectomy. Ann Surg Oncol 2019;26:2952–8.ArticlePubMedPDF
- 37. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Total thyroidectomy versus lobectomy in conventional papillary thyroid microcarcinoma: analysis of 8,676 patients at a single institution. Surgery 2017;161:485–92.ArticlePubMed
- 38. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid 2018;28:1587–94.ArticlePubMed
- 39. Kim SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Differences in the recurrence and survival of patients with symptomatic and asymptomatic papillary thyroid carcinoma: an observational study of 11,265 person-years of follow-up. Thyroid 2016;26:1472–9.ArticlePubMed
- 40. Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab 2015;100:4434–40.ArticlePubMedPMC
- 41. Houten R, Fleeman N, Kotas E, Boland A, Lambe T, Duarte R. A systematic review of health state utility values for thyroid cancer. Qual Life Res 2021;30:675–702.ArticlePubMedPMCPDF
- 42. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30.ArticlePubMed
- 43. Kim SY, Kim SM, Chang H, Kim BW, Lee YS, Chang HS, et al. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and sorafenib in Korea. Front Endocrinol (Lausanne) 2019;10:384.ArticlePubMedPMC
- 44. Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and costing in cost-effectiveness analysis, 1974-2018. Pharmacoeconomics 2020;38:1135–45.ArticlePubMedPMCPDF
- 45. Jones E, Epstein D, Garcia-Mochon L. A procedure for deriving formulas to convert transition rates to probabilities for multistate Markov models. Med Decis Making 2017;37:779–89.ArticlePubMedPMCPDF
- 46. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022;25:10–31.ArticlePubMed
- 47. Hammer MM, Kong CY. Cost-effectiveness of follow-up ultrasound for incidental thyroid nodules on CT. AJR Am J Roentgenol 2022;218:615–22.ArticlePubMed
- 48. Ernst R. Indirect costs and cost-effectiveness analysis. Value Health 2006;9:253–61.ArticlePubMed
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite