Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Sestrin2 Regulates Beneficial β3-Adrenergic Receptor-Mediated Effects Observed in Inguinal White Adipose Tissue and Soleus Muscle
- Min Jeong Park, Joo Won Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Hwan-Jin Hwang, Hye Jin Yoo
- Endocrinol Metab. 2022;37(3):552-557. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1421
- 2,653 View
- 107 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
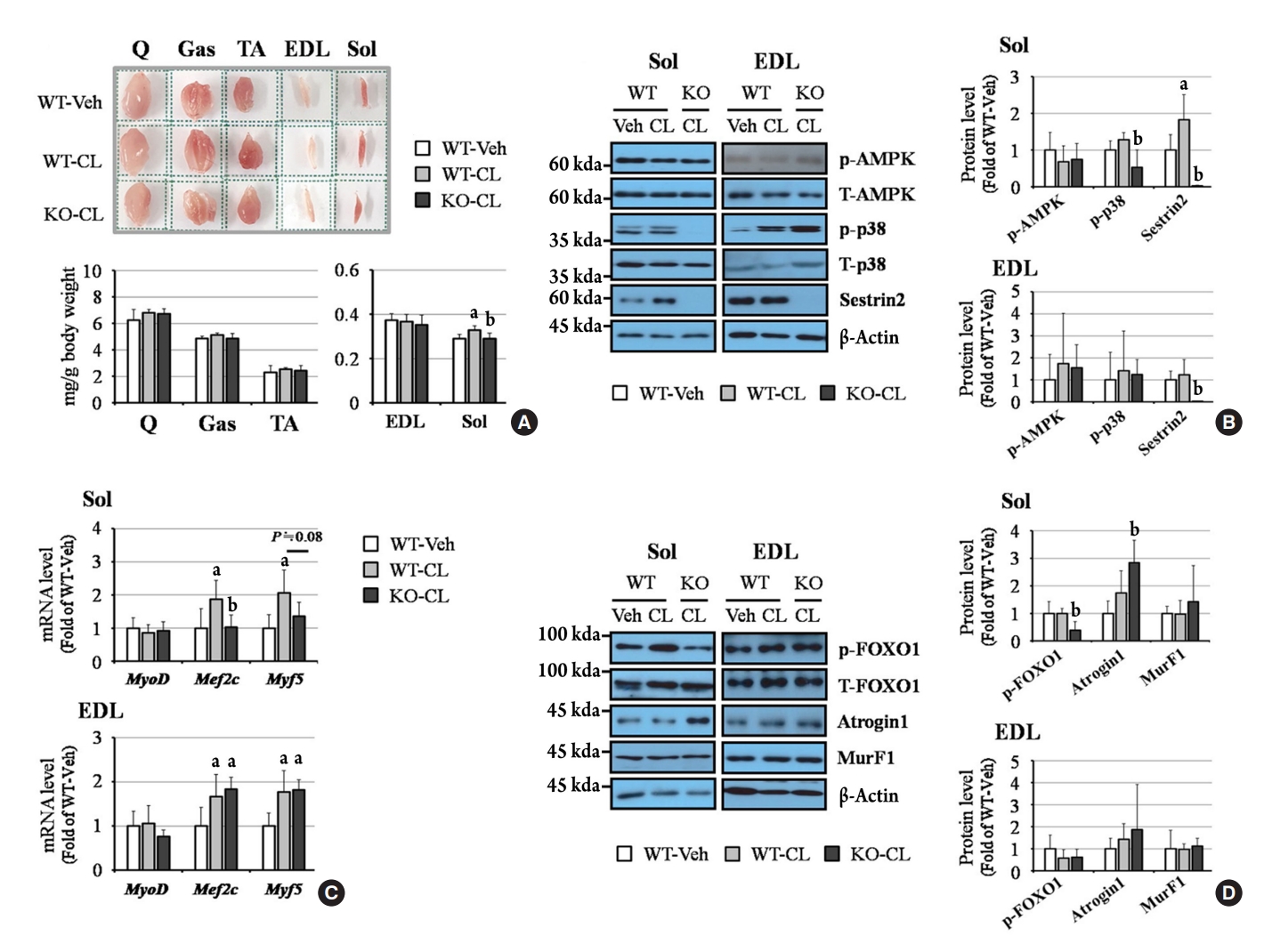
ePub - Sestrin2, a well-known adenosine monophosphate-activated protein kinase (AMPK) regulator, plays a protective role against metabolic stress. The β3-adrenergic receptor (β3AR) induces fat browning and inhibits muscle atrophy in an AMPK-dependent manner. However, no prior research has examined the relationship of sestrin2 with β3AR in body composition changes. In this study, CL 316,243 (CL), a β3AR agonist, was administered to wild-type and sestrin2-knockout (KO) mice for 2 weeks, and fat and muscle tissues were harvested. CL induced AMPK phosphorylation, expression of brown-fat markers, and mitochondrial biogenesis, which resulted in the reduction of lipid droplet size in inguinal white adipose tissue (iWAT). These effects were not observed in sestrin2-KO mice. In CL-treated soleus muscle, sestrin2-KO was related to decreased myogenic gene expression and increased levels of muscle atrophy-related molecules. Our results suggest that sestrin2 is associated with beneficial β3AR-mediated changes in body composition, especially in iWAT and in the soleus.
-
Citations
Citations to this article as recorded by- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation
Yufeng Qian, Lian Chen, Beibei Gao, Xianhua Ye
Clinical and Experimental Hypertension.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation

- Clinical Study
- The Role of Circulating Slit2, the One of the Newly Batokines, in Human Diabetes Mellitus
- Yea Eun Kang, Sorim Choung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
- Endocrinol Metab. 2017;32(3):383-388. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.383
- 3,866 View
- 52 Download
- 19 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Slit2 is a new secreted protein from adipose tissue that improves glucose hemostasis in mice; however, there is no study about the serum levels and precise role of Slit2 in human. The aim of this study is to explore the serum level of Slit2 in human, and to identify the role of Slit2 in diabetes mellitus (DM).
Methods The participants of this study consist of 38 subjects with newly diagnosed DM, and 75 healthy subjects as a control group. Serum Slit2 levels were measured using an enzyme-linked immunosorbent assay. Relationship between circulating Slit2 and diabetic related factors was investigated in diabetic group compared with non-diabetic group. Additionally, the correlations between the serum level of Slit2 and diverse metabolic parameters were analyzed.
Results Circulating Slit2 level was more decreased in diabetic group than in control group, but there was no significant difference statistically. Interestingly, serum levels of Slit2 were significantly negatively correlated to the serum concentrations of fasting glucose (coefficient
r =–0.246,P =0.008), the serum concentrations of postprandial glucose (coefficientr =–0.233,P =0.017), and glycosylated hemoglobin (HbA1c; coefficientr =–0.357,P <0.001).Conclusion From our study, the first report of circulating Slit2 levels in human, circulating Slit2 level significantly negatively correlated with serum glucose and HbA1c. Our results suggest that the circulating Slit2 may play a role in maintainence of glucose homeostasis in human, even though exact contribution and mechanism are not yet known.
-
Citations
Citations to this article as recorded by- Brown adipose tissue-derived metabolites and their role in regulating metabolism
Khanyisani Ziqubu, Phiwayinkosi V. Dludla, Sihle E. Mabhida, Babalwa U. Jack, Susanne Keipert, Martin Jastroch, Sithandiwe E. Mazibuko-Mbeje
Metabolism.2024; 150: 155709. CrossRef - An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications
Zeinab Ghesmati, Mohsen Rashid, Shabnam Fayezi, Frank Gieseler, Effat Alizadeh, Masoud Darabi
Reviews in Endocrine and Metabolic Disorders.2024; 25(2): 279. CrossRef - The integrated bioinformatic analysis identifies immune microenvironment-related potential biomarkers for patients with gestational diabetes mellitus
Jie-ling Chen, Hui-fang Dai, Xin-chen Kan, Jie Wu, Hong-Wu Chen
Frontiers in Immunology.2024;[Epub] CrossRef - Adipokines from white adipose tissue in regulation of whole body energy homeostasis
Bijayashree Sahu, Naresh C. Bal
Biochimie.2023; 204: 92. CrossRef - The Role of Slit-2 in Gestational Diabetes Mellitus and Its Effect on Pregnancy Outcome
Yan Wang, Shihua Zhao, Wei Peng, Ying Chen, Jingwei Chi, Kui Che, Yangang Wang
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Heartwarming Effect of Brown Adipose Tissue
Kelsey M. Pinckard, Kristin I. Stanford
Molecular Pharmacology.2022; 102(1): 39. CrossRef - New players of the adipose secretome: Therapeutic opportunities and challenges
Laetitia Coassolo, Niels Banhos Dannieskiold-Samsøe, Meng Zhao, Hobson Allen, Katrin J. Svensson
Current Opinion in Pharmacology.2022; 67: 102302. CrossRef - Serum CD14 concentration is associated with obesity and insulin resistance in non-diabetic individuals
Yea Eun Kang, Kyong Hye Joung, Ji Min Kim, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Journal of International Medical Research.2022; 50(10): 030006052211300. CrossRef - Brown/Beige adipose tissues and the emerging role of their secretory factors in improving metabolic health: The batokines
Bilal Ahmad, Muhammad Sufyan Vohra, Mansab Ali Saleemi, Christopher J. Serpell, Isabel Lim Fong, Eng Hwa Wong
Biochimie.2021; 184: 26. CrossRef - Thermogenic Fat: Development, Physiological Function, and Therapeutic Potential
Bruna B. Brandão, Ankita Poojari, Atefeh Rabiee
International Journal of Molecular Sciences.2021; 22(11): 5906. CrossRef - Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism
Camilla Scheele, Christian Wolfrum
Endocrine Reviews.2020; 41(1): 53. CrossRef - Development of a Cell-Based Assay for the Detection of Neutralizing Antibodies to PF-06730512 Using Homogenous Time-Resolved Fluorescence
Michael Luong, Ying Wang, Stephen P. Berasi, Janet E. Buhlmann, Hongying Yang, Boris Gorovits
The AAPS Journal.2020;[Epub] CrossRef - Brown and beige fat: From molecules to physiology and pathophysiology
Stefania Carobbio, Anne-Claire Guénantin, Isabella Samuelson, Myriam Bahri, Antonio Vidal-Puig
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(1): 37. CrossRef - Serum R-Spondin 1 Is a New Surrogate Marker for Obesity and Insulin Resistance
Yea Eun Kang, Ji Min Kim, Hyon-Seung Yi, Kyong Hye Joung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Diabetes & Metabolism Journal.2019; 43(3): 368. CrossRef - Deletion of Robo4 prevents high‐fat diet‐induced adipose artery and systemic metabolic dysfunction
Tam T. T. Phuong, Ashley E. Walker, Grant D. Henson, Daniel R. Machin, Dean Y. Li, Anthony J. Donato, Lisa A. Lesniewski
Microcirculation.2019;[Epub] CrossRef - Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs
Min-Woo Lee, Mihye Lee, Kyoung-Jin Oh
Journal of Clinical Medicine.2019; 8(6): 854. CrossRef - The role of brown and beige adipose tissue in glycaemic control
Katarina Klepac, Anastasia Georgiadi, Matthias Tschöp, Stephan Herzig
Molecular Aspects of Medicine.2019; 68: 90. CrossRef
- Brown adipose tissue-derived metabolites and their role in regulating metabolism


 KES
KES

 First
First Prev
Prev



