Search
- Page Path
- HOME > Search
Review Article
- Calcium & bone metabolism
- Acquired Forms of Fibroblast Growth Factor 23-Related Hypophosphatemic Osteomalacia
- Nobuaki Ito, Naoko Hidaka, Hajime Kato
- Endocrinol Metab. 2024;39(2):255-261. Published online March 11, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1908
- 1,502 View
- 79 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
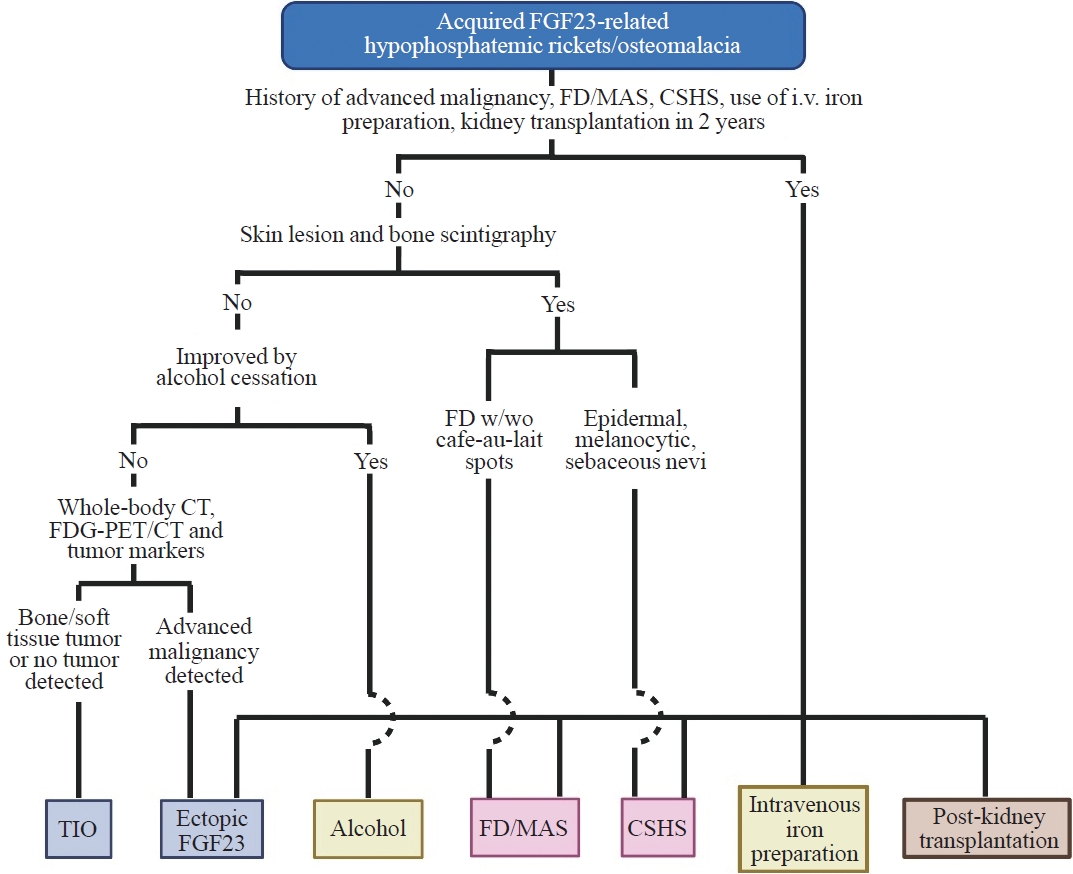
ePub - Fibroblast growth factor 23 (FGF23) is a pivotal humoral factor for the regulation of serum phosphate levels and was first identified in patients with autosomal dominant hypophosphatemic rickets and tumor-induced osteomalacia (TIO), the most common form of acquired FGF23-related hypophosphatemic rickets/osteomalacia (FGF23rHR). After the identification of FGF23, many other inherited and acquired forms of FGF23rHR were reported. In this review article, the detailed features of each acquired FGF23rHR are discussed, including TIO, ectopic FGF23 syndrome with malignancy, fibrous dysplasia/McCune-Albright syndrome, Schimmelpenning-Feuerstein-Mims syndrome/cutaneous skeletal hypophosphatemia syndrome, intravenous iron preparation-induced FGF23rHR, alcohol consumption-induced FGF23rHR, and post-kidney transplantation hypophosphatemia. Then, an approach for the differential diagnosis and therapeutic options for each disorder are concisely introduced. Currently, the majority of endocrinologists might only consider TIO when encountering patients with acquired FGF23rHR; an adequate differential diagnosis can reduce medical costs and invasive procedures such as positron emission tomography/computed tomography and venous sampling to identify FGF23-producing tumors. Furthermore, some acquired FGF23rHRs, such as intravenous iron preparation/alcohol consumption-induced FGF23rHR, require only cessation of drugs or alcohol to achieve full recovery from osteomalacia.

Original Articles
- Mutational Analysis of Gsalpha Protein in Fibrous dysplasia of the Bone.
- Sang Youl Rhee, Jeong Taek Woo, Gwanpyo Koh, Seungjoon Oh, Sung Woon Kim, Jin Woo Kim, Young Seol Kim, Yong Koo Park
- J Korean Endocr Soc. 2005;20(2):142-147. Published online April 1, 2005
- DOI: https://doi.org/10.3803/jkes.2005.20.2.142
- 1,497 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Fibrous dysplasia of the bone(FD) is a benign fibrous bone lesion which usually involves the long bones of the extremities. FD may be asymptomatic, but often leads to bone deformity and pathological fracture. The disease is caused by a somatic mutation in the Gsalpha protein, which is responsible for intracellular signal transduction. METHODS: Mutations in the GNAS1 gene, which codes for Gsalpha protein, was investigated in 34 patients with monostotic and polyostotic FD and McCune-Albright syndrome. DNA was extracted from formalin-fixed, paraffin embedded bone tissues, and exons 8 and 9 of the GNAS1 gene amplified using a polymerase chain reaction(PCR). Subsequently, plasmid cloning and DNA sequencing analysis were performed. RESULTS: The PCR was successfully performed in 5 patients with monostotic FD. However, the sequencing analysis failed to identify any significant point mutations in exons 8 or 9 of GNAS1. Nevertheless, 3 point mutations were observed in the intron of the GNAS1 gene in 2 samples. CONCLUSION: In addition to the previously known somatic mutations of the GNAS1 gene, this study suggests that fibrous dysplasia of the bone might be associated with another point mutations of the GNAS1 gene

- A Case of Atypical McCune-Albright Syndrome Associated with Hyperthyroidism and Hypersecretion of Growth Hormone.
- Moon Bin You, Ki Hoon Kang, Byung Soo Lee, Eun Ha Chae, Myung Chan Kim, Jae Il Jung, Sun Hee Park, Hyo Jin Lee, Seok Tae Jung
- J Korean Endocr Soc. 2003;18(4):426-432. Published online August 1, 2003
- 981 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - McCune-Albright syndrome is characterized by polyostotic fibrous dysplasia, Caf -au-lait pigmentation and precocious puberty or other endocrinopathy. It can be caused by substitution of His, Cys or Gly for Arg 201st amino acid of the Gs protein subunit. The case of a 32-year-old woman, with atypical McCune-Albright syndrome, is reported. She had no skin lesion or precocity puberty. The polyostotic fibrous dysplasia was examined by a simple radiological image and whole body scan. She developed hyperthyroidism, with a multinodular toxic goiter. No thyroid related autoantibodies were detected. The cause of hyperthyroidism was thought to be a non- autoimmune thyroid hyperfunction. The level of growth hormone was not suppressed by oral glucose load. After a bromocriptine suppression test, the level of growth hormone decreased. There was no mass in the pituitary gland on a sellar MRI. A case of atypical McCune-Albright syndrome, including hyperthyroidism and hypersecretion of growth hormone, is reported.

Case Report
- A Case of McCunt-Albright Syndrome Associated with Acremegaly and Fibrous Dysplasia.
- Jung Guk Kim, Sung Woo Ha, Sang Won Chung, Seong Mo Koo, Jae Tae Lee, Kyu Bo Lee, Yong Sun Kim, Sam Kwon, Bo Wan Kim
- J Korean Endocr Soc. 1998;13(1):108-114. Published online January 1, 2001
- 1,093 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Acromegaly and hyperprolactinemia have been described in association with polyostotic fibrous dysplasia. The pathogenic mechanisms of this endocrinopathy are not clear. We experienced a 19-year-old male with hypersecretion of GH, hyperprolactinemia and fibrous dysplasia. He was referred for evaluation of suspected acromegaly. He had no skin pigmentation. Plasma GH, PRL, somatomedin-C, LH, FSH, testosterone, estradiol, progesterone, T3, T4, TSH and cortisol were measured. Among those, the levels of plasma GH, PRL and somatomedin-C were high. Serum alkaline phosphatase was increased. OGTT did not suppress plasma OH concentration and GH showed paradoxical response to TRH and LHRH. GH was suppressed after a test-dose of somatastatin and bromocriptine. Brain MRI demonstrated a mass lesion in sella turcica and another mass lesions in nasal cavity and posterior occipital bone. Whole body bone scan revealed increased uptake in skull, nasal bone, both 9th posterior rib, both femurs, both tibias, left scapular and pelvic bone. These fmdings were consistent with bone tumor such as fibrous dysplasia. We report a case with incomplete MeCune-Albright syndrome including acromegaly, hyperprolactinemia and polyostotic fibrous dysplasia.


 KES
KES
 First
First Prev
Prev



