Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
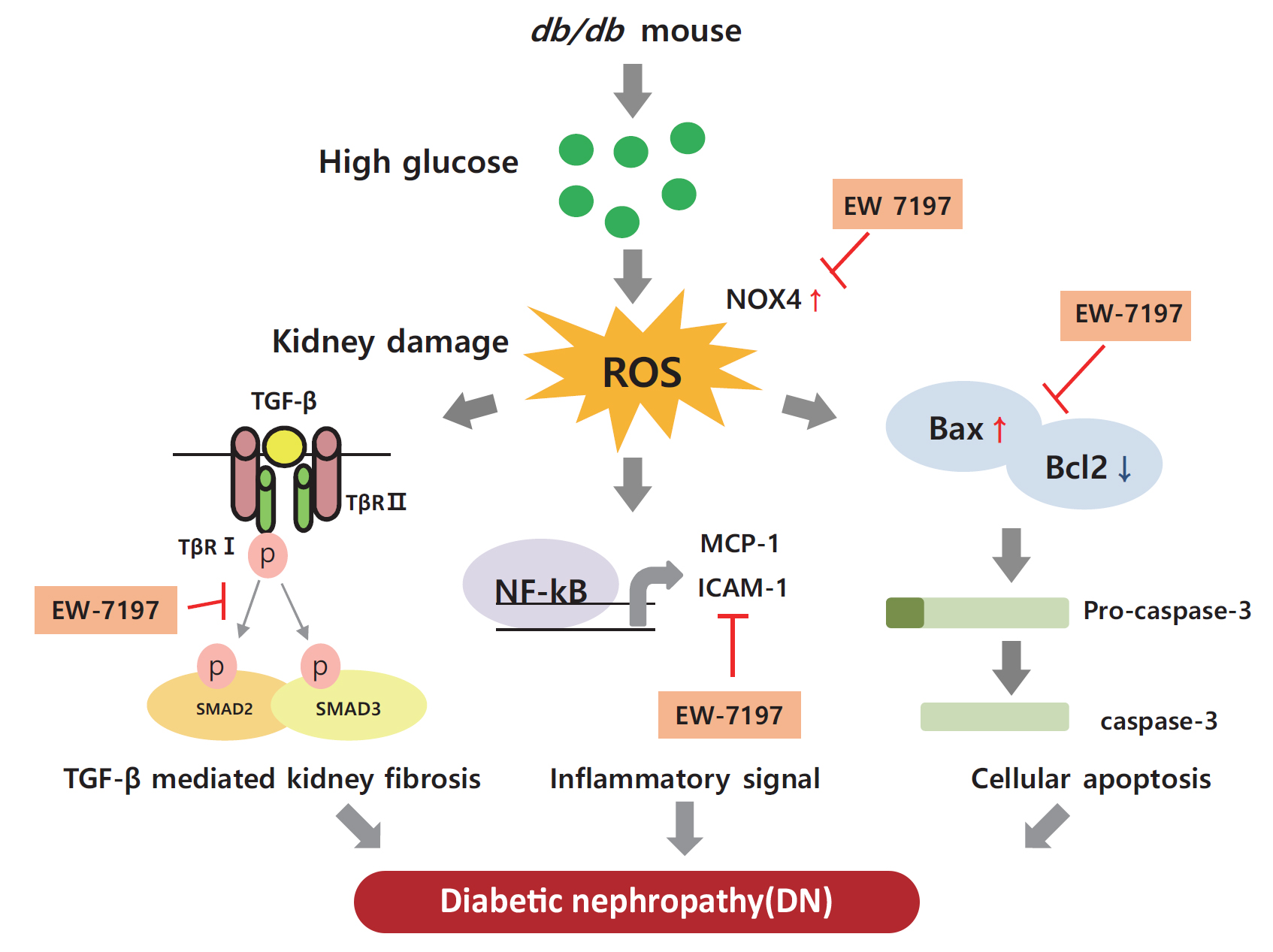
- EW-7197 Attenuates the Progression of Diabetic Nephropathy in db/db Mice through Suppression of Fibrogenesis and Inflammation
- Kyung Bong Ha, Weerapon Sangartit, Ah Reum Jeong, Eun Soo Lee, Hong Min Kim, Soyeon Shim, Upa Kukongviriyapan, Dae-Kee Kim, Eun Young Lee, Choon Hee Chung
- Endocrinol Metab. 2022;37(1):96-111. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1305
- 4,008 View
- 181 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Diabetic nephropathy (DN) is characterized by albuminuria and accumulation of extracellular matrix (ECM) in kidney. Transforming growth factor-β (TGF-β) plays a central role in promoting ECM accumulation. We aimed to examine the effects of EW-7197, an inhibitor of TGF-β type 1 receptor kinase (ALK5), in retarding the progression of DN, both in vivo, using a diabetic mouse model (db/db mice), and in vitro, in podocytes and mesangial cells.
Methods
In vivo study: 8-week-old db/db mice were orally administered EW-7197 at a dose of 5 or 20 mg/kg/day for 10 weeks. Metabolic parameters and renal function were monitored. Glomerular histomorphology and renal protein expression were evaluated by histochemical staining and Western blot analyses, respectively. In vitro study: DN was induced by high glucose (30 mM) in podocytes and TGF-β (2 ng/mL) in mesangial cells. Cells were treated with EW-7197 (500 nM) for 24 hours and the mechanism associated with the attenuation of DN was investigated.
Results
Enhanced albuminuria and glomerular morphohistological changes were observed in db/db compared to that of the nondiabetic (db/m) mice. These alterations were associated with the activation of the TGF-β signaling pathway. Treatment with EW-7197 significantly inhibited TGF-β signaling, inflammation, apoptosis, reactive oxygen species, and endoplasmic reticulum stress in diabetic mice and renal cells.
Conclusion
EW-7197 exhibits renoprotective effect in DN. EW-7197 alleviates renal fibrosis and inflammation in diabetes by inhibiting downstream TGF-β signaling, thereby retarding the progression of DN. Our study supports EW-7197 as a therapeutically beneficial compound to treat DN. -
Citations
Citations to this article as recorded by- TGF-β signaling in health, disease, and therapeutics
Ziqin Deng, Tao Fan, Chu Xiao, He Tian, Yujia Zheng, Chunxiang Li, Jie He
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols
Qi Jin, Tongtong Liu, Yuan Qiao, Donghai Liu, Liping Yang, Huimin Mao, Fang Ma, Yuyang Wang, Liang Peng, Yongli Zhan
Frontiers in Immunology.2023;[Epub] CrossRef - Beneficial Effects of a Curcumin Derivative and Transforming Growth Factor-β Receptor I Inhibitor Combination on Nonalcoholic Steatohepatitis
Kyung Bong Ha, Eun Soo Lee, Na Won Park, Su Ho Jo, Soyeon Shim, Dae-Kee Kim, Chan Mug Ahn, Choon Hee Chung
Diabetes & Metabolism Journal.2023; 47(4): 500. CrossRef
- TGF-β signaling in health, disease, and therapeutics

- Endocrine Research
- Irisin Regulates the Functions of Hepatic Stellate Cells
- Hanh Nguyen Dong, So Young Park, Cong Thuc Le, Dae-Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2020;35(3):647-655. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.658

- 6,514 View
- 180 Download
- 11 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Hepatic stellate cells (HSCs) are known to play a fundamental role in the progression of liver fibrosis. Once HSCs are activated, they are involved in proliferation, migration, and contractility which are characteristics of liver fibrogenesis. Recent studies have shown that irisin, a myokine secreted during physical exercise, has a protective effect in various metabolic diseases, especially in renal fibrosis. However, whether irisin is involved in HSC activation and other processes associated with liver fibrosis has not yet been investigated. In this study, we reveal the role of irisin in HSC activation as well as in proliferation, migration, and contractile properties of HSCs in vitro.
Methods
LX-2 cells, immortalized human HSCs, were treated with transforming growth factor beta 1 (TGF-β1), a core regulator of HSC fibrosis, with or without irisin, and markers of the aforementioned processes were analyzed. Further, an inflammatory response was stimulated with TGF-β1 and lipopolysaccharide (LPS) in combination with irisin and the expression of cytokines was measured.
Results
Recombinant irisin significantly suppressed the expression of TGF-β1-stimulated fibrosis markers including alpha-smooth muscle actin and collagen type 1 alpha 1 and prevented the TGF-β1-induced proliferation, migration, and contractility of LX-2 cells. Additionally, irisin ameliorated the production of interleukin-6 (IL-6) and IL-1β induced by TGF-β1 and LPS treatments.
Conclusion
These findings suggested that irisin potently improved the progression of hepatic fibrosis by regulating HSC activation, proliferation, migration, contractility, and HSC-mediated production of inflammatory cytokine. -
Citations
Citations to this article as recorded by- Potential role of irisin in digestive system diseases
Yueming Zhang, Linxian Zhao, Huan Gao, Jinghui Zhai, Yanqing Song
Biomedicine & Pharmacotherapy.2023; 166: 115347. CrossRef - Potential role of irisin in lung diseases and advances in research
Hongna Dong, Xuejiao Lv, Peng Gao, Yuqiu Hao
Frontiers in Pharmacology.2023;[Epub] CrossRef - Stem bark of Fraxinus rhynchophylla ameliorates the severity of pancreatic fibrosis by regulating the TGF-β/Smad signaling pathway
Ji-Won Choi, Joon Yeon Shin, Ziqi Zhou, Dong-Uk Kim, Bitna Kweon, Hyuncheol Oh, Youn-Chul Kim, Ho-Joon Song, Gi-Sang Bae, Sung-Joo Park
Journal of Investigative Medicine.2022; 70(5): 1285. CrossRef - Circadian rhythms and cancers: the intrinsic links and therapeutic potentials
Li Zhou, Zhe Zhang, Edouard Nice, Canhua Huang, Wei Zhang, Yong Tang
Journal of Hematology & Oncology.2022;[Epub] CrossRef - Kinsenoside alleviates inflammation and fibrosis in experimental NASH mice by suppressing the NF-κB/NLRP3 signaling pathway
Yan-fang Deng, Qian-qian Xu, Tian-qi Chen, Jia-xiong Ming, Ya-fen Wang, Li-na Mao, Jia-jun Zhou, Wei-guang Sun, Qun Zhou, Hong Ren, Yong-hui Zhang
Phytomedicine.2022; 104: 154241. CrossRef - The potential role of FNDC5/irisin in various liver diseases: awakening the sleeping beauties
Xiaoyu Wang, Lihong Mao, Chaoqun Li, Yangyang Hui, Zihan Yu, Mingyu Sun, Yifan Li, Gaoyue Guo, Wanting Yang, Binxin Cui, Xiaofei Fan, Chao Sun
Expert Reviews in Molecular Medicine.2022;[Epub] CrossRef - The Effects of Irisin on the Interaction between Hepatic Stellate Cell and Macrophage in Liver Fibrosis
Dinh Vinh Do, So Young Park, Giang Thi Nguyen, Dae Hee Choi, Eun-Hee Cho
Endocrinology and Metabolism.2022; 37(4): 620. CrossRef - Hepatic Steatosis Contributes to the Development of Muscle Atrophy via Inter-Organ Crosstalk
Kenneth Pasmans, Michiel E. Adriaens, Peter Olinga, Ramon Langen, Sander S. Rensen, Frank G. Schaap, Steven W. M. Olde Damink, Florian Caiment, Luc J. C. van Loon, Ellen E. Blaak, Ruth C. R. Meex
Frontiers in Endocrinology.2021;[Epub] CrossRef - Physiopathology of Lifestyle Interventions in Non-Alcoholic Fatty Liver Disease (NAFLD)
David Carneros, Guillermo López-Lluch, Matilde Bustos
Nutrients.2020; 12(11): 3472. CrossRef
- Potential role of irisin in digestive system diseases

- Bone Metabolism
- Recent Topics in Fibrodysplasia Ossificans Progressiva
- Takenobu Katagiri, Sho Tsukamoto, Yutaka Nakachi, Mai Kuratani
- Endocrinol Metab. 2018;33(3):331-338. Published online September 18, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.3.331
- 5,079 View
- 79 Download
- 19 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Fibrodysplasia ossificans progressiva (FOP) is a rare genetic disease that is characterized by the formation of heterotopic bone tissues in soft tissues, such as skeletal muscle, ligament, and tendon. It is difficult to remove such heterotopic bones via internal medicine or invasive procedures. The identification of activin A receptor, type I (
ACVR1 )/ALK2 gene mutations associated with FOP has allowed the genetic diagnosis of FOP. TheACVR1 /ALK2 gene encodes the ALK2 protein, which is a transmembrane kinase receptor in the transforming growth factor-β family. The relevant mutations activate intracellular signalingin vitro and induce heterotopic bone formationin vivo . Activin A is a potential ligand that activates mutant ALK2 but not wild-type ALK2. Various types of small chemical and biological inhibitors of ALK2 signaling have been developed to establish treatments for FOP. Some of these are in clinical trials in patients with FOP.-
Citations
Citations to this article as recorded by- How Activin A Became a Therapeutic Target in Fibrodysplasia Ossificans Progressiva
Dushyanth Srinivasan, Martin Arostegui, Erich J. Goebel, Kaitlin N. Hart, Senem Aykul, John B. Lees-Shepard, Vincent Idone, Sarah J. Hatsell, Aris N. Economides
Biomolecules.2024; 14(1): 101. CrossRef - Fibrodysplasia Ossificans Progressiva Mimics Generalized Dystonia Disorder: A Case Report
Seraj Makkawi, Osama Khojah, Reema Abualnaja, Abdulaziz Qashqari, Nawaf A Alahmadi, Abdullatif G Bshnaq, Abdulrahman Alharthi, Hashem H Al-Hashemi, Aiman M Shawli
Cureus.2023;[Epub] CrossRef - Exploration of marine natural resources in Indonesia and development of efficient strategies for the production of microbial halogenated metabolites
Hiroyuki Yamazaki
Journal of Natural Medicines.2022; 76(1): 1. CrossRef - A Novel De Novo Frameshift Pathogenic Variant in the FAM111B Resulting in Progressive Osseous Heteroplasia Phenotype
Anna Ryabets-Lienhard, Panadeekarn Panjawatanan, Kyle Vogt, Jianling Ji, Senta Georgia, Pisit Pitukcheewanont
Calcified Tissue International.2022; 112(4): 518. CrossRef - Inhibitory effects of sesquiterpene lactones from the Indonesian marine sponge Lamellodysidea cf. herbacea on bone morphogenetic protein-induced osteoblastic differentiation
Satoshi Ohte, Hiroyuki Yamazaki, Ohgi Takahashi, Henki Rotinsulu, Defny S. Wewengkang, Deiske A. Sumilat, Delfly B. Abdjul, Wilmar Maarisit, Magie M. Kapojos, Huiping Zhang, Fumiaki Hayashi, Michio Namikoshi, Takenobu Katagiri, Hiroshi Tomoda, Ryuji Uchid
Bioorganic & Medicinal Chemistry Letters.2021; 35: 127783. CrossRef - Genomic Context and Mechanisms of the ACVR1 Mutation in Fibrodysplasia Ossificans Progressiva
Roberto Ravazzolo, Renata Bocciardi
Biomedicines.2021; 9(2): 154. CrossRef - New insights on fibrodysplasia ossificans progressiva: discussion of an autoptic case report and brief literature review
Vittorio Bolcato, Claudia Carelli, Silvia Damiana Visonà, Marcella Reguzzoni, Maja Di Rocco, Alessandra Radogna, Livio Pietro Tronconi, Matteo Moretti
Intractable & Rare Diseases Research.2021; 10(2): 136. CrossRef - Accumulated Knowledge of Activin Receptor-Like Kinase 2 (ALK2)/Activin A Receptor, Type 1 (ACVR1) as a Target for Human Disorders
Takenobu Katagiri, Sho Tsukamoto, Mai Kuratani
Biomedicines.2021; 9(7): 736. CrossRef - Cytoskeleton Reorganization in EndMT—The Role in Cancer and Fibrotic Diseases
Wojciech Michał Ciszewski, Marta Ewelina Wawro, Izabela Sacewicz-Hofman, Katarzyna Sobierajska
International Journal of Molecular Sciences.2021; 22(21): 11607. CrossRef - Alendronate disturbs femoral growth due to changes during immunolocalization of transforming growth factor-β1 and bone morphogenetic protein-2 in epiphyseal plate
Juliana Souza Vieira, Emanuelle Juliana Cunha, Juliana Feltrin de Souza, Luis Henrique Koeler Chaves, Jessica Lakes de Souza, Allan Fernando Giovanini
World Journal of Experimental Medicine.2020; 10(1): 1. CrossRef - ALK2: A Therapeutic Target for Fibrodysplasia Ossificans Progressiva and Diffuse Intrinsic Pontine Glioma
Katsuhiko Sekimata, Tomohiro Sato, Naoki Sakai
Chemical and Pharmaceutical Bulletin.2020; 68(3): 194. CrossRef - Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases
Riko Nishimura, Kenji Hata, Yoshifumi Takahata, Tomohiko Murakami, Eriko Nakamura, Maki Ohkawa, Lerdluck Ruengsinpinya
International Journal of Molecular Sciences.2020; 21(4): 1340. CrossRef - Design of primers for direct sequencing of nine coding exons in the human ACVR1 gene
Masaru Matsuoka, Sho Tsukamoto, Yuta Orihara, Rieko Kawamura, Mai Kuratani, Nobuhiko Haga, Kenji Ikebuchi, Takenobu Katagiri
Bone.2020; 138: 115469. CrossRef - A new diketopiperazine-like inhibitor of bone morphogenetic protein-induced osteoblastic differentiation produced by marine-derived Aspergillus sp. BFM-0085
Satoshi Ohte, Takehiro Shiokawa, Nobuhiro Koyama, Takenobu Katagiri, Chiaki Imada, Hiroshi Tomoda
The Journal of Antibiotics.2020; 73(8): 554. CrossRef - Penicillic Acid Congener, a New Inhibitor of BMP-Induced Alkaline Phosphatase Activity in Myoblasts, Produced by the Fungus Penicillium sp. BF-0343
Nobuhiro Koyama, Yasuhiro Otoguro, Satoshi Ohte, Takenobu Katagiri, Hiroshi Tomoda
Natural Product Communications.2020; 15(9): 1934578X2094265. CrossRef - Fibrodysplasia ossificans progressiva: current concepts from bench to bedside
Arun-Kumar Kaliya-Perumal, Tom J. Carney, Philip W. Ingham
Disease Models & Mechanisms.2020;[Epub] CrossRef - Clinical Aspects and Current Therapeutic Approaches for FOP
Hiroshi Kitoh
Biomedicines.2020; 8(9): 325. CrossRef - Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates
Hiroyuki Yamazaki, Satoshi Ohte, Henki Rotinsulu, Defny S. Wewengkang, Deiske A. Sumilat, Delfly B. Abdjul, Wilmar Maarisit, Magie M. Kapojos, Michio Namikoshi, Takenobu Katagiri, Hiroshi Tomoda, Ryuji Uchida
Marine Drugs.2020; 18(12): 606. CrossRef - Propranolol and ascorbic acid in control of fibrodysplasia ossificans progressiva flare-ups due to accidental falls
Durval Batista Palhares, Deborah Ribeiro Nascimento, Marilene Garcia Palhares, Suzana Lopes Bomfim Balaniuc, Liane de Rosso Giuliani, Paula Cristhina Niz Xavier, José Mauro Goulart Brum, Fabiana Alves, Francisco Oliveira Vieira, Elaine Maria Souza-Fagunde
Intractable & Rare Diseases Research.2019; 8(1): 24. CrossRef - Late-onset fibrodysplasia ossificans progressiva with atypical presentation: A case report
Conor M. Cunningham, J. Matthew Royeca, Samuel W. King, Hemant Pandit
Case Reports in Women's Health.2019; 23: e00134. CrossRef - Fibrodysplasia ossificans progressiva: lessons learned from a rare disease
Gulseren Akyuz, Kardelen Gencer-Atalay, Pinar Ata
Current Opinion in Pediatrics.2019; 31(6): 716. CrossRef - Discovery of Heterotopic Bone-Inducing Activity in Hard Tissues and the TGF-β Superfamily
Takenobu Katagiri, Sho Tsukamoto, Yutaka Nakachi, Mai Kuratani
International Journal of Molecular Sciences.2018; 19(11): 3586. CrossRef
- How Activin A Became a Therapeutic Target in Fibrodysplasia Ossificans Progressiva

- Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Kwi-Hyun Bae, Jung Beom Seo, Yun-A Jung, Hye-Young Seo, Sun Hee Kang, Hui-Jeon Jeon, Jae Man Lee, Sungwoo Lee, Jung-Guk Kim, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
- Endocrinol Metab. 2017;32(1):115-123. Published online February 28, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.115
- 4,843 View
- 77 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Renal tubulointerstitial fibrosis is a common feature of the final stage of nearly all cause types of chronic kidney disease. Although classic peroxisome proliferator-activated receptor γ (PPARγ) agonists have a protective effect on diabetic nephropathy, much less is known about their direct effects in renal fibrosis. This study aimed to investigate possible beneficial effects of lobeglitazone, a novel PPARγ agonist, on renal fibrosis in mice.
Methods We examined the effects of lobeglitazone on renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO) induced renal fibrosis mice. We further defined the role of lobeglitazone on transforming growth factor (TGF)-signaling pathways in renal tubulointerstitial fibrosis through
in vivo andin vitro study.Results Through hematoxylin/eosin and sirius red staining, we observed that lobeglitazone effectively attenuates UUO-induced renal atrophy and fibrosis. Immunohistochemical analysis in conjunction with quantitative reverse transcription polymerase chain reaction and Western blot analysis revealed that lobeglitazone treatment inhibited UUO-induced upregulation of renal Smad-3 phosphorylation, α-smooth muscle actin, plasminogen activator inhibitor 1, and type 1 collagen.
In vitro experiments with rat mesangial cells and NRK-49F renal fibroblast cells suggested that the effects of lobeglitazone on UUO-induced renal fibrosis are mediated by inhibition of the TGF-β/Smad signaling pathway.Conclusion The present study demonstrates that lobeglitazone has a protective effect on UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of non-diabetic origin renal disease.
-
Citations
Citations to this article as recorded by- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis
Shiyan Jian, Kang Yang, Lingna Zhang, Limeng Zhang, Zhongquan Xin, Chaoyu Wen, Shansong He, Jinping Deng, Baichuan Deng
Food Frontiers.2023; 4(1): 262. CrossRef - Druggability of lipid metabolism modulation against renal fibrosis
Yuan-yuan Chen, Xiao-guang Chen, Sen Zhang
Acta Pharmacologica Sinica.2022; 43(3): 505. CrossRef - Lobeglitazone attenuates fibrosis in corneal fibroblasts by interrupting TGF-beta-mediated Smad signaling
Selikem Nuwormegbe, Na-Young Park, Sun Woong Kim
Graefe's Archive for Clinical and Experimental Ophthalmology.2022; 260(1): 149. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
Endocrinology and Metabolism.2021; 36(5): 1095. CrossRef - Protocol for a preclinical systematic review and meta-analysis of pharmacological targeting of peroxisome proliferator-activated receptors in experimental renal injury
William P Martin, Yeong H D Chuah, Emer Conroy, Alison L Reynolds, Conor Judge, Francisco J López-Hernández, Carel W le Roux, Neil G Docherty
BMJ Open Science.2021;[Epub] CrossRef - Stevioside inhibits unilateral ureteral obstruction‐induced kidney fibrosis and upregulates renal PPARγ expression in mice
Wei Shen, Ke Fan, Ying Zhao, Junyan Zhang, Meilin Xie
Journal of Food Biochemistry.2020;[Epub] CrossRef - FBW7 Regulates the Autophagy Signal in Mesangial Cells Induced by High Glucose
Chenlin Gao, Fang Fan, Jiao Chen, Yang Long, Shi Tang, Chunxia Jiang, Yong Xu
BioMed Research International.2019; 2019: 1. CrossRef - Treatment with Lobeglitazone Attenuates Hepatic Steatosis in Diet-Induced Obese Mice
Sorim Choung, Kyong Hye Joung, Bo Ram You, Sang Ki Park, Hyun Jin Kim, Bon Jeong Ku
PPAR Research.2018; 2018: 1. CrossRef - VCE‐004.3, a cannabidiol aminoquinone derivative, prevents bleomycin‐induced skin fibrosis and inflammation through PPARγ‐ and CB2 receptor‐dependent pathways
Carmen del Rio, Irene Cantarero, Belén Palomares, María Gómez‐Cañas, Javier Fernández‐Ruiz, Carolina Pavicic, Adela García‐Martín, Maria Luz Bellido, Rafaela Ortega‐Castro, Carlos Pérez‐Sánchez, Chary López‐Pedrera, Giovanni Appendino, Marco A Calzado, Ed
British Journal of Pharmacology.2018; 175(19): 3813. CrossRef - EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis
Adela García-Martín, Martín Garrido-Rodríguez, Carmen Navarrete, Carmen del Río, María L. Bellido, Giovanni Appendino, Marco A. Calzado, Eduardo Muñoz
Biochemical Pharmacology.2018; 157: 304. CrossRef - Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
Kyoung Min Kim, Hyun-Jin Jin, Seo Yeon Lee, Hyo Jin Maeng, Gha Young Lee, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang, Soo Lim
Endocrinology and Metabolism.2017; 32(3): 389. CrossRef - Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
Soo Lim, Kyoung Min Kim, Sin Gon Kim, Doo Man Kim, Jeong-Taek Woo, Choon Hee Chung, Kyung Soo Ko, Jeong Hyun Park, Yongsoo Park, Sang Jin Kim, Hak Chul Jang, Dong Seop Choi
Diabetes & Metabolism Journal.2017; 41(5): 377. CrossRef
- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis

- Bone Metabolism
- Dissecting Tumor-Stromal Interactions in Breast Cancer Bone Metastasis
- Yibin Kang
- Endocrinol Metab. 2016;31(2):206-212. Published online May 13, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.206
- 4,989 View
- 54 Download
- 34 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Bone metastasis is a frequent occurrence in breast cancer, affecting more than 70% of late stage cancer patients with severe complications such as fracture, bone pain, and hypercalcemia. The pathogenesis of osteolytic bone metastasis depends on cross-communications between tumor cells and various stromal cells residing in the bone microenvironment. Several growth factor signaling pathways, secreted micro RNAs (miRNAs) and exosomes are functional mediators of tumor-stromal interactions in bone metastasis. We developed a functional genomic approach to systemically identified molecular pathways utilized by breast cancer cells to engage the bone stroma in order to generate osteolytic bone metastasis. We showed that elevated expression of vascular cell adhesion molecule 1 (VCAM1) in disseminated breast tumor cells mediates the recruitment of pre-osteoclasts and promotes their differentiation to mature osteoclasts during the bone metastasis formation. Transforming growth factor β (TGF-β) is released from bone matrix upon bone destruction, and signals to breast cancer to further enhance their malignancy in developing bone metastasis. We furthered identified Jagged1 as a TGF-β target genes in tumor cells that engaged bone stromal cells through the activation of Notch signaling to provide a positive feedback to promote tumor growth and to activate osteoclast differentiation. Substantially change in miRNA expression was observed in osteoclasts during their differentiation and maturation, which can be exploited as circulating biomarkers of emerging bone metastasis and therapeutic targets for the treatment of bone metastasis. Further research in this direction may lead to improved diagnosis and treatment strategies for bone metastasis.
-
Citations
Citations to this article as recorded by- Osteoclast Cancer Cell Metabolic Cross-talk Confers PARP Inhibitor Resistance in Bone Metastatic Breast Cancer
Huijuan Fan, Zhanao Xu, Ke Yao, Bingxin Zheng, Yuan Zhang, Xuxiang Wang, Tengjiang Zhang, Xuan Li, Haitian Hu, Bin Yue, Zeping Hu, Hanqiu Zheng
Cancer Research.2024; 84(3): 449. CrossRef - Bone Marrow Mesenchymal Stem Cells Restrain the Migration and Invasion of Breast Cancer Cells by Up-Regulating miR-2158 and Inactivating RAI2/NLRP3 Pathway
Meiyu Xu, Shen Ye, Zhiqiang Tang, Shuai Gong
Journal of Biomaterials and Tissue Engineering.2023; 13(1): 162. CrossRef - Association of RANKL and EGFR gene expression with bone metastases in patients with metastatic non-small cell lung cancer
Anita J.W.M. Brouns, Lizza E.L. Hendriks, Iris J. Robbesom-van den Berge, Annemariek J.H.M. Driessen, Guido M.J.M. Roemen, Britt L.J. van Herpen, Zoë Dekkers, Bas Heitzer, Daphne J.G. Leunissen, Laura Moonen, Ragnar Lunde, Marcel Westenend, Marjolein van
Frontiers in Oncology.2023;[Epub] CrossRef - BMAL1 induces colorectal cancer metastasis by stimulating exosome secretion
Pengjuan Dong, Yaping Wang, Yutong Liu, Chunting Zhu, Jiaxin Lin, Ruizhe Qian, Luchun Hua, Chao Lu
Molecular Biology Reports.2022; 49(1): 373. CrossRef - Chemokines network in bone metastasis: Vital regulators of seeding and soiling
Gunjan Sharma, Ramesh Pothuraju, Ranjana Kumari Kanchan, Surinder Kumar Batra, Jawed Akhtar Siddiqui
Seminars in Cancer Biology.2022; 86: 457. CrossRef - The Signaling Pathways Associated With Breast Cancer Bone Metastasis
Xuelian Song, Changran Wei, Xiangqi Li
Frontiers in Oncology.2022;[Epub] CrossRef - Effects of 8-week noncontinuous aerobic exercise on the levels of CCL2, CCL5, and their respective receptors in female BALB/C mice suffering from breast cancer
Mehrnoosh Esmailiyan, Mehdi Kargarfard, Fahimeh Esfarjani, Golnaz Vaseghi
International Journal of Preventive Medicine.2022; 13(1): 55. CrossRef - Non‐coding RNAs in bone remodelling and bone metastasis: Mechanisms of action and translational relevance
Margherita Puppo, Hanna Taipaleenmäki, Eric Hesse, Philippe Clézardin
British Journal of Pharmacology.2021; 178(9): 1936. CrossRef - Sympathetic activity in breast cancer and metastasis: partners in crime
Francisco Conceição, Daniela M. Sousa, Joana Paredes, Meriem Lamghari
Bone Research.2021;[Epub] CrossRef - Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis
Shenglong Li, Wei Wang
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Bone marrow niches in the regulation of bone metastasis
Fenfang Chen, Yujiao Han, Yibin Kang
British Journal of Cancer.2021; 124(12): 1912. CrossRef - Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer
Kerui Wu, Jiamei Feng, Feng Lyu, Fei Xing, Sambad Sharma, Yin Liu, Shih-Ying Wu, Dan Zhao, Abhishek Tyagi, Ravindra Pramod Deshpande, Xinhong Pei, Marco Gabril Ruiz, Hiroyuki Takahashi, Shunsuke Tsuzuki, Takahiro Kimura, Yin-yuan Mo, Yusuke Shiozawa, Ravi
Nature Communications.2021;[Epub] CrossRef - Circulating Osteocalcin-Positive Cells as a Novel Diagnostic Biomarker for Bone Metastasis in Breast Cancer Patients
Kyung-Hun Lee, Kyoung Jin Lee, Tae-Yong Kim, Febby Hutomo, Hyun Jin Sun, Gi Jeong Cheon, Serk In Park, Sun Wook Cho, Seock-Ah Im
Journal of Bone and Mineral Research.2020; 35(10): 1838. CrossRef - Polymer nanomedicines
Jindřich Kopeček, Jiyuan Yang
Advanced Drug Delivery Reviews.2020; 156: 40. CrossRef - Osteolytic metastasis in breast cancer: effective prevention strategies
Chandi C Mandal
Expert Review of Anticancer Therapy.2020; 20(9): 797. CrossRef - The Tumor Microenvironment of Primitive and Metastatic Breast Cancer: Implications for Novel Therapeutic Strategies
Giovanni Zarrilli, Gianluca Businello, Maria Vittoria Dieci, Silvia Paccagnella, Valentina Carraro, Rocco Cappellesso, Federica Miglietta, Gaia Griguolo, Valentina Guarneri, Marcello Lo Mele, Matteo Fassan
International Journal of Molecular Sciences.2020; 21(21): 8102. CrossRef - Identification and validation of DOCK4 as a potential biomarker for risk of bone metastasis development in patients with early breast cancer
Jules A Westbrook, Steven L Wood, David A Cairns, Kathryn McMahon, Renu Gahlaut, Helene Thygesen, Mike Shires, Stephanie Roberts, Helen Marshall, Maria R Oliva, Mark J Dunning, Andrew M Hanby, Peter J Selby, Valerie Speirs, Georgia Mavria, Robert E Colema
The Journal of Pathology.2019; 247(3): 381. CrossRef - The Relationship between Exosomes and Cancer: Implications for Diagnostics and Therapeutics
Wendy W. Weston, Timothy Ganey, H. Thomas Temple
BioDrugs.2019; 33(2): 137. CrossRef - In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer
Montemagno, Dumas, Cavaillès, Ahmadi, Bacot, Debiossat, Soubies, Djaïleb, Leenhardt, Leiris, Dufies, Pagès, Hernot, Devoogdt, Perret, Riou, Fagret, Ghezzi, Broisat
Cancers.2019; 11(7): 1039. CrossRef - Notch and breast cancer metastasis: Current knowledge, new sights and targeted therapy (Review)
Yu Zhang, Zi‑Yan Xie, Xuan‑Tong Guo, Xing‑Hua Xiao, Li‑Xia Xiong
Oncology Letters.2019;[Epub] CrossRef - NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche
Dongsheng Wang, Chenglong Zhao, Liangliang Gao, Yao Wang, Xin Gao, Liang Tang, Kun Zhang, Zhenxi Li, Jing Han, Jianru Xiao
Journal of Bone Oncology.2018; 13: 91. CrossRef - Placental exosomes: A proxy to understand pregnancy complications
Jin Jin, Ramkumar Menon
American Journal of Reproductive Immunology.2018;[Epub] CrossRef - Understanding the Bone in Cancer Metastasis
Jaime Fornetti, Alana L Welm, Sheila A Stewart
Journal of Bone and Mineral Research.2018; 33(12): 2099. CrossRef - Role of Tumor-Derived Chemokines in Osteolytic Bone Metastasis
Salvatore J. Coniglio
Frontiers in Endocrinology.2018;[Epub] CrossRef - The role of exosomes in cancer metastasis
Teresa Bernadette Steinbichler, József Dudás, Herbert Riechelmann, Ira-Ida Skvortsova
Seminars in Cancer Biology.2017; 44: 170. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Impaired Bone Matrix Alignment Induced by Breast Cancer Metastasis
Aiko Sekita, Aira Matsugaki, Takayoshi Nakano
Journal of the Japan Institute of Metals.2017; 81(6): 308. CrossRef - SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts
Zhan Xu, Matthew B. Greenblatt, Guang Yan, Heng Feng, Jun Sun, Sutada Lotinun, Nicholas Brady, Roland Baron, Laurie H. Glimcher, Weiguo Zou
Nature Communications.2017;[Epub] CrossRef - Bone Microenvironment and Role of Rank-Rankl-Opg in Breast Cancer Metastasis in Bone
Hongwei Zhang
Journal of Cancer Prevention & Current Research.2017;[Epub] CrossRef - Tumor–Stroma Interactions in Bone Metastasis: Molecular Mechanisms and Therapeutic Implications
Hanqiu Zheng, Wenyang Li, Yibin Kang
Cold Spring Harbor Symposia on Quantitative Biology.2016; 81: 151. CrossRef - Breast cancer cells obtain an osteomimetic featureviaepithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction
Cong-Cong Tan, Gui-Xi Li, Li-Duan Tan, Xin Du, Xiao-Qing Li, Rui He, Qing-Shan Wang, Yu-Mei Feng
Oncotarget.2016; 7(48): 79688. CrossRef - Heterotypic models of osteosarcoma recapitulate tumor heterogeneity and biological behavior
Milcah C. Scott, Hirotaka Tomiyasu, John R. Garbe, Ingrid Cornax, Clarissa Amaya, M Gerard O'Sullivan, Subbaya Subramanian, Brad A. Bryan, Jaime F. Modiano
Disease Models & Mechanisms.2016;[Epub] CrossRef - Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
Hyo Min Jeong, Sun Wook Cho, Serk In Park
Endocrinology and Metabolism.2016; 31(4): 485. CrossRef
- Osteoclast Cancer Cell Metabolic Cross-talk Confers PARP Inhibitor Resistance in Bone Metastatic Breast Cancer


 KES
KES

 First
First Prev
Prev



