Funded articles
- Page Path
- HOME > BROWSE ARTICLES > Funded articles
Review Article
- Calcium & bone metabolism
- Skeletal Senescence with Aging and Type 2 Diabetes
- Joshua Nicholas Farr
- Endocrinol Metab. 2023;38(3):295-301. Published online June 14, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1727
- Funded: National Institutes of Health

- 2,678 View
- 127 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Osteoporosis and type 2 diabetes (T2D) are common diseases that often coexist. While both of these diseases are associated with poor bone quality and increased fracture risk, their pathogenesis of increased fracture risk differs and is multifactorial. Mounting evidence now indicates that key fundamental mechanisms that are central to both aging and energy metabolism exist. Importantly, these mechanisms represent potentially modifiable therapeutic targets for interventions that could prevent or alleviate multiple complications of osteoporosis and T2D, including poor bone quality. One such mechanism that has gained increasing momentum is senescence, which is a cell fate that contributes to multiple chronic diseases. Accumulating evidence has established that numerous boneresident cell types become susceptible to cellular senescence with old age. Recent work also demonstrates that T2D causes the premature accumulation of senescent osteocytes during young adulthood, at least in mice, although it remains to be seen which other bone-resident cell types become senescent with T2D. Given that therapeutically removing senescent cells can alleviate age-related bone loss and T2D-induced metabolic dysfunction, it will be important in future studies to rigorously test whether interventions that eliminate senescent cells can also alleviate skeletal dysfunction in context of T2D, as it does with aging.
-
Citations
Citations to this article as recorded by- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Cellular Neuroscience.2024;[Epub] CrossRef - The synergistic effect of diabetes mellitus and osteoporosis on the all-cause mortality: a cohort study of an American population
Weihua Li, Siyu Xie, Shengdong Zhong, Liting Lan
Frontiers in Endocrinology.2024;[Epub] CrossRef - Identification of systemic biomarkers and potential drug targets for age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Aging Neuroscience.2024;[Epub] CrossRef
- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration

Original Articles
- Miscellaneous
- Association between N-Terminal Prohormone Brain Natriuretic Peptide and Decreased Skeletal Muscle Mass in a Healthy Adult Population: A Cross-Sectional Study
- Tae Kyung Yoo, Marie Yung-Chen Wu, Moon Soo Kim, Mi-Yeon Lee, Yong-Taek Lee, Kyung Jae Yoon, Chul-Hyun Park
- Endocrinol Metab. 2023;38(2):269-276. Published online March 13, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1588
- Funded: National Research Foundation of Korea, Ministry of Science and ICT
- 1,814 View
- 77 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Although an inverse association between the N-terminal prohormone brain natriuretic peptide (NT-proBNP) and obesity exists, only few major studies have assessed the association between NT-proBNP levels and skeletal muscle mass in asymptomatic healthy adults. Therefore, this cross-sectional study was conducted.
Methods
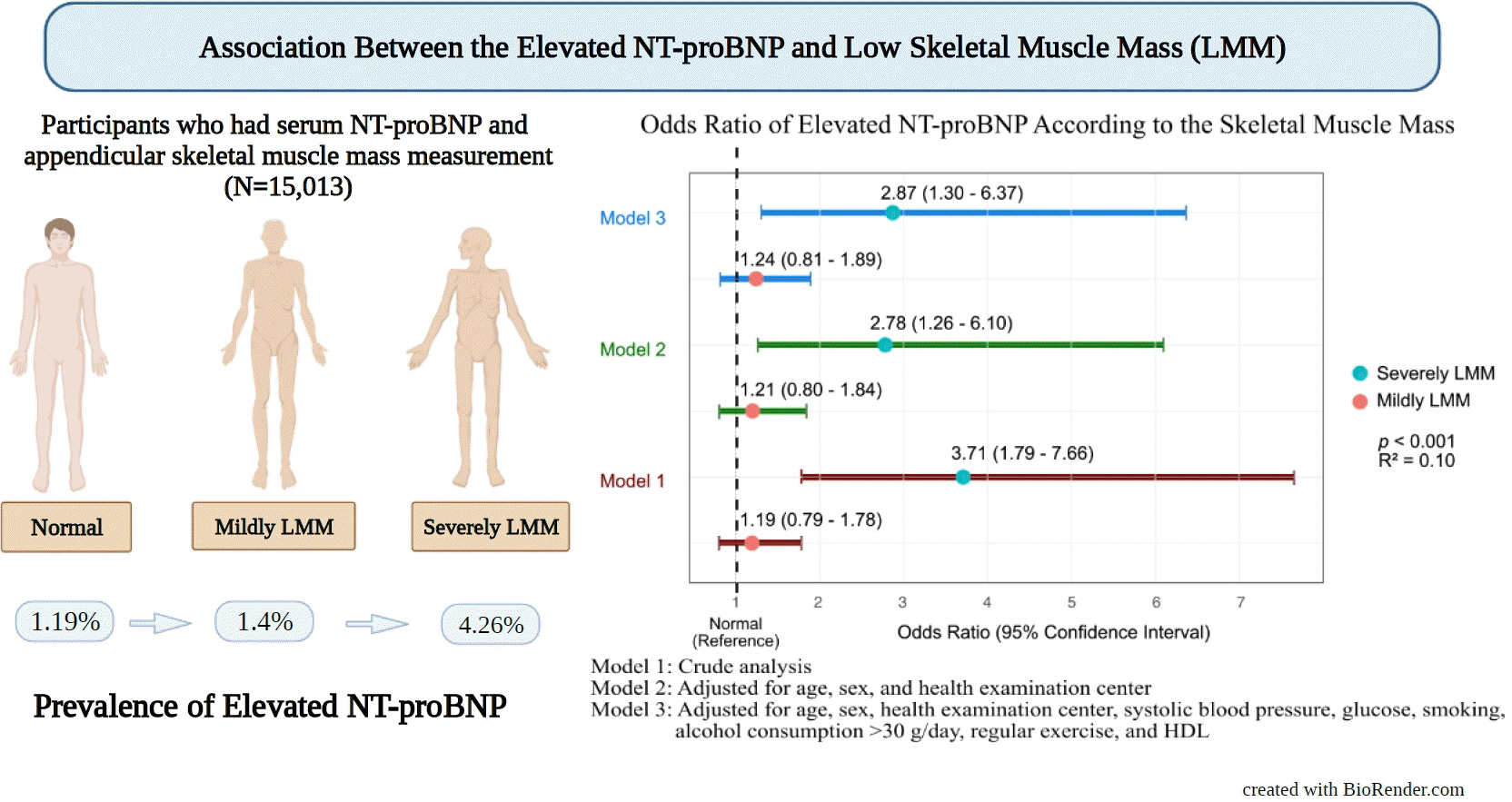
We assessed participants who underwent health examinations at Kangbuk Samsung Hospital in South Korea from January 2012 to December 2019. Appendicular skeletal muscle mass was measured using a bioelectrical impedance analyzer, and the skeletal muscle mass index (SMI) was calculated. Participants were divided into the control, mildly low skeletal muscle mass (LMM) (−2 standard deviation [SD] < SMI ≤−1 [SD]), and severely LMM groups (SD ≤−2) based on their SMI. The association between elevated NT-proBNP level (≥125 pg/mL) and skeletal muscle mass was assessed using multivariable logistic regression analysis with adjustment for confounding factors.
Results
This study enrolled 15,013 participants (mean age, 37.52±9.52; men, 54.24%; control, n=12,827; mildly LMM, n=1,998; severely LMM, n=188). Prevalence of elevated NT-proBNP was higher in mildly and severely LMM groups than in the control group (control, 1.19%; mildly LMM, 1.4%; severely LMM, 4.26%; P=0.001). The adjusted odds ratio (OR) of elevated NT-proBNP was significantly higher in severely LMM (OR, 2.87; 95% confidence interval [CI], 1.3 to 6.37) than in control (OR, 1.00; reference) or mildly LMM groups (OR, 1.24; 95% CI, 0.81 to 1.89).
Conclusion
Our results showed that NT-proBNP elevation were more prevalent in participants with LMM. In addition, our study showed an association between skeletal muscle mass and NT-proBNP level in a relatively young and healthy adult population.

- Calcium & bone metabolism
- Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
- Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
- Endocrinol Metab. 2023;38(2):260-268. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1663
- Funded: Catholic University of Korea, Uijeongbu St. Mary’s Hospital Clinical Research Laboratory Foundation
- 1,750 View
- 109 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Persistence with denosumab in male patients has not been adequately investigated, although poor denosumab persistence is associated with a significant risk of rebound vertebral fractures.
Methods
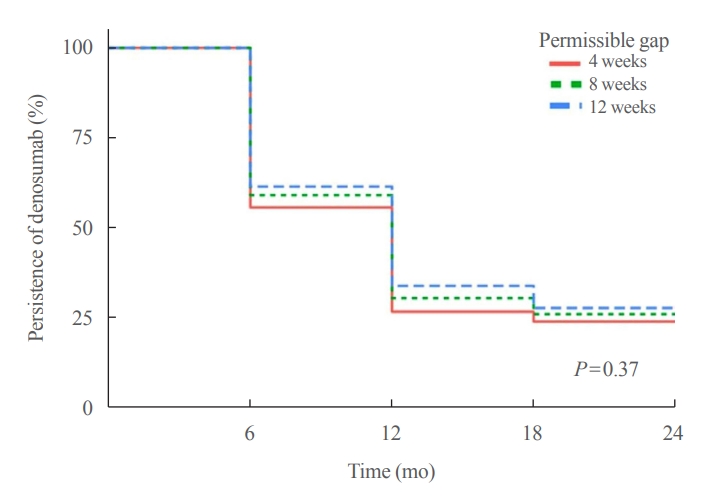
We retrospectively evaluated 294 Korean male osteoporosis patients treated with denosumab at three medical centers and examined their persistence with four doses of denosumab injection over 24 months of treatment. Persistence was defined as the extent to which a patient adhered to denosumab treatment in terms of the prescribed interval and dose, with a permissible gap of 8 weeks. For patients who missed their scheduled treatment appointment(s) during the follow-up period (i.e., no-shows), Cox proportional regression analysis was conducted to explore the factors associated with poor adherence. Several factors were considered, such as age, prior anti-osteoporotic drug use, the treatment provider’s medical specialty, the proximity to the medical center, and financial burdens of treatment.
Results
Out of 294 male patients, 77 (26.2%) completed all four sequential rounds of the denosumab treatment. Out of 217 patients who did not complete the denosumab treatment, 138 (63.6%) missed the scheduled treatment(s). Missing treatment was significantly associated with age (odds ratio [OR], 1.03), prior bisphosphonate use (OR, 0.76), and prescription by non-endocrinologists (OR, 2.24). Denosumab was stopped in 44 (20.3%) patients due to medical errors, in 24 (11.1%) patients due to a T-score improvement over –2.5, and in five (2.3%) patients due to expected dental procedures.
Conclusion
Our study showed that only one-fourth of Korean male osteoporosis patients were fully adherent to 24 months of denosumab treatment. -
Citations
Citations to this article as recorded by- Denosumab
Reactions Weekly.2023; 1963(1): 206. CrossRef
- Denosumab

- Adrenal gland
Big Data Articles (National Health Insurance Service Database) - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
- Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
- Endocrinol Metab. 2023;38(2):253-259. Published online March 21, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1607
- Funded: Korean Endocrinology Society
- 2,584 View
- 103 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The severity of coronavirus disease 2019 (COVID-19) among patients with long-term glucocorticoid treatment (LTGT) has not been established. We aimed to evaluate the association between LTGT and COVID-19 prognosis.
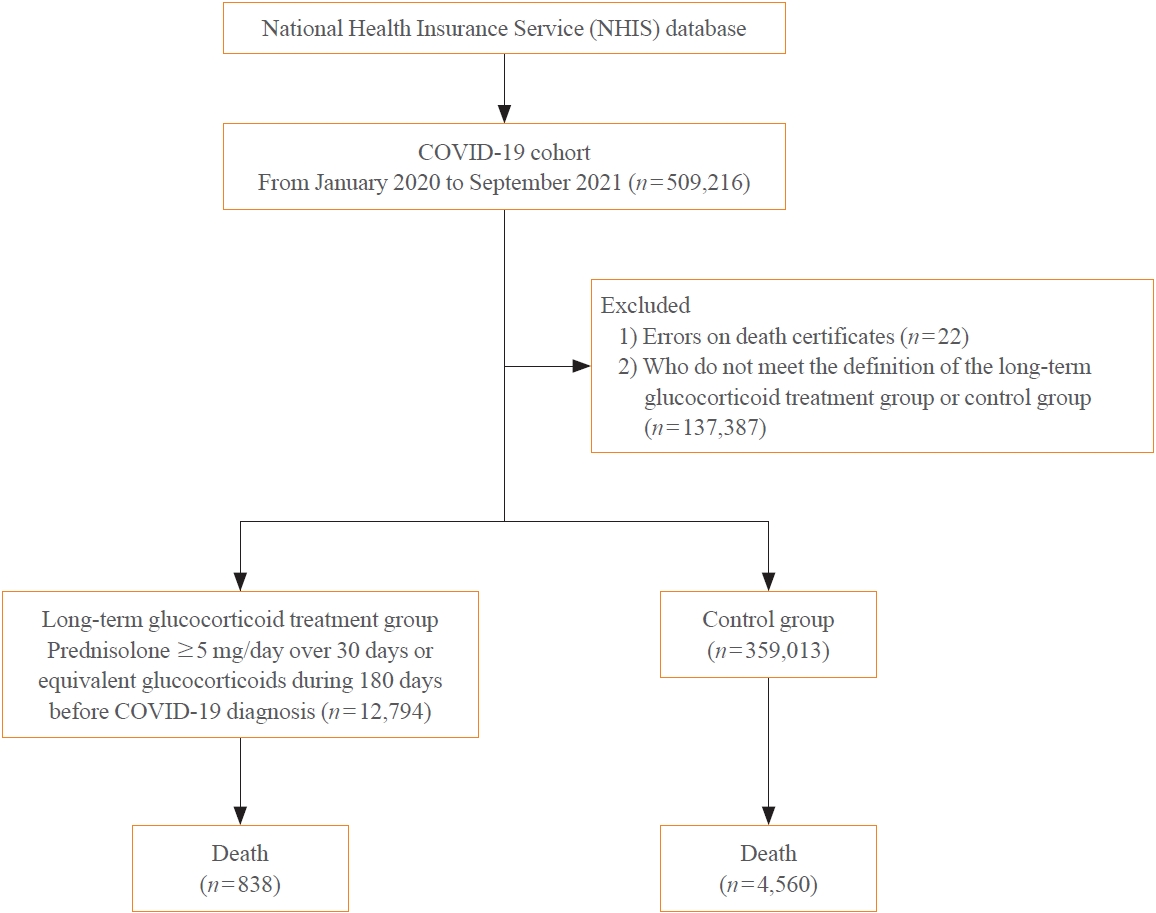
Methods
A Korean nationwide cohort database of COVID-19 patients between January 2019 and September 2021 was used. LTGT was defined as exposure to at least 150 mg of prednisolone (≥5 mg/day and ≥30 days) or equivalent glucocorticoids 180 days before COVID-19 infection. The outcome measurements were mortality, hospitalization, intensive care unit (ICU) admission, length of stay, and mechanical ventilation.
Results
Among confirmed patients with COVID-19, the LTGT group (n=12,794) was older and had a higher proportion of comorbidities than the control (n=359,013). The LTGT group showed higher in-hospital, 30-day, and 90-day mortality rates than the control (14.0% vs. 2.3%, 5.9% vs. 1.1%, and 9.9% vs. 1.8%, respectively; all P<0.001). Except for the hospitalization rate, the length of stay, ICU admission, and mechanical ventilation proportions were significantly higher in the LTGT group than in the control (all P<0.001). Overall mortality was higher in the LTGT group than in the control group, and the significance remained in the fully adjusted model (odds ratio [OR], 5.75; 95% confidence interval [CI], 5.31 to 6.23) (adjusted OR, 1.82; 95% CI, 1.67 to 2.00). The LTGT group showed a higher mortality rate than the control within the same comorbidity score category.
Conclusion
Long-term exposure to glucocorticoids increased the mortality and severity of COVID-19. Prevention and early proactive measures are inevitable in the high-risk LTGT group with many comorbidities. -
Citations
Citations to this article as recorded by- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
Eun-Hee Cho
Endocrinology and Metabolism.2023; 38(2): 223. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy

- Diabetes, obesity and metabolism
Big Data Articles (National Health Insurance Service Database) - Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
- Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
- Endocrinol Metab. 2023;38(2):245-252. Published online April 5, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1662
- Funded: Korean Endocrine Society
- 2,245 View
- 120 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
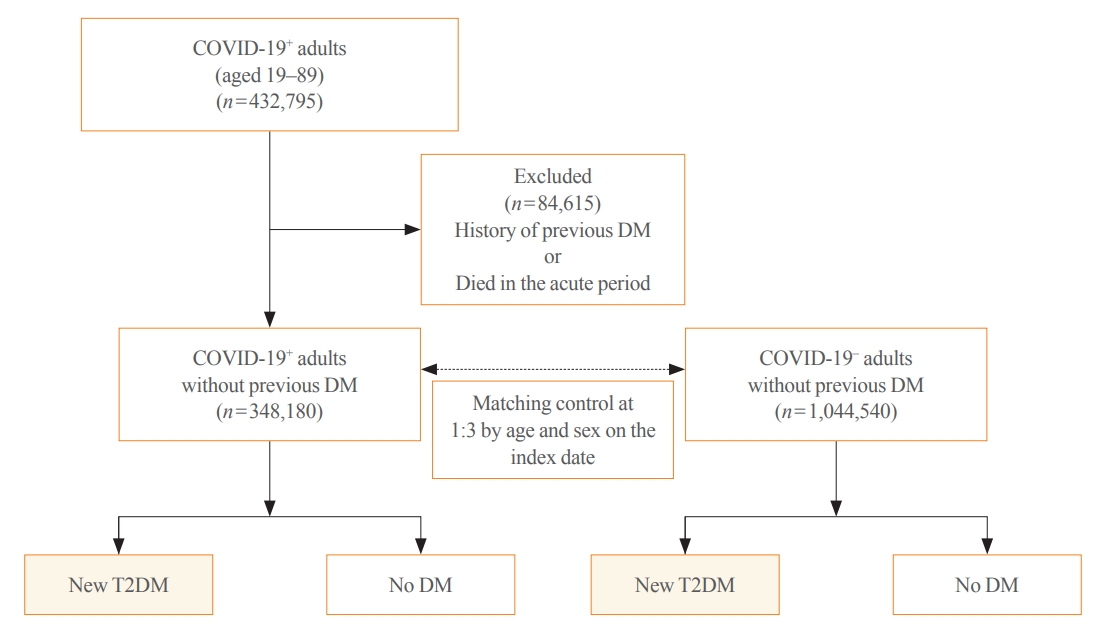
Methods
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
Conclusion
COVID-19 may increase the risk of developing T2DM beyond the acute period. The higher the severity of COVID-19 in the acute phase, the higher the risk of newly diagnosed T2DM. Therefore, T2DM should be included as a component of managing long-term COVID-19. -
Citations
Citations to this article as recorded by- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review
Anca Pantea Stoian, Ioana-Cristina Bica, Teodor Salmen, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez,
Diabetes Therapy.2024; 15(1): 33. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review

- Diabetes, obesity and metabolism
- Inhibition of Fatty Acid β-Oxidation by Fatty Acid Binding Protein 4 Induces Ferroptosis in HK2 Cells Under High Glucose Conditions
- Jiasi Chen, Keping Wu, Yan Lei, Mingcheng Huang, Lokyu Cheng, Hui Guan, Jiawen Lin, Ming Zhong, Xiaohua Wang, Zhihua Zheng
- Endocrinol Metab. 2023;38(2):226-244. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1604
- Funded: Sanming Project of Medicine in Shenzhen, National Nature Science Foundation of China, The Shenzhen Science and Technology Innovation Committee of Guangdong Province of China
- 3,564 View
- 178 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
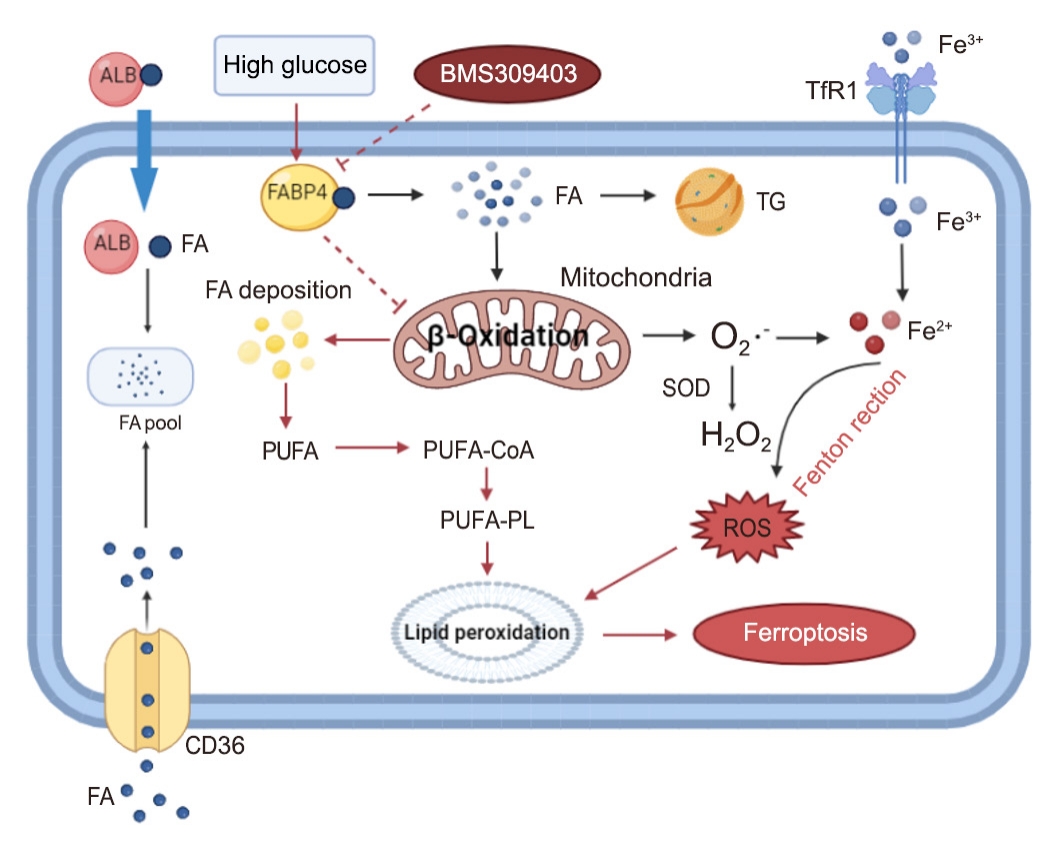
Ferroptosis, which is caused by an iron-dependent accumulation of lipid hydroperoxides, is a type of cell death linked to diabetic kidney disease (DKD). Previous research has shown that fatty acid binding protein 4 (FABP4) is involved in the regulation of ferroptosis in diabetic retinopathy. The present study was constructed to explore the role of FABP4 in the regulation of ferroptosis in DKD.
Methods
We first detected the expression of FABP4 and proteins related to ferroptosis in renal biopsies of patients with DKD. Then, we used a FABP4 inhibitor and small interfering RNA to investigate the role of FABP4 in ferroptosis induced by high glucose in human renal proximal tubular epithelial (HG-HK2) cells.
Results
In kidney biopsies of DKD patients, the expression of FABP4 was elevated, whereas carnitine palmitoyltransferase-1A (CP-T1A), glutathione peroxidase 4, ferritin heavy chain, and ferritin light chain showed reduced expression. In HG-HK2 cells, the induction of ferroptosis was accompanied by an increase in FABP4. Inhibition of FABP4 in HG-HK2 cells changed the redox state, sup-pressing the production of reactive oxygen species, ferrous iron (Fe2+), and malondialdehyde, increasing superoxide dismutase, and reversing ferroptosis-associated mitochondrial damage. The inhibition of FABP4 also increased the expression of CPT1A, reversed lipid deposition, and restored impaired fatty acid β-oxidation. In addition, the inhibition of CPT1A could induce ferroptosis in HK2 cells.
Conclusion
Our results suggest that FABP4 mediates ferroptosis in HG-HK2 cells by inhibiting fatty acid β-oxidation. -
Citations
Citations to this article as recorded by- Fatty Acid Binding Protein-4 Silencing Inhibits Ferroptosis to Alleviate Lipopolysaccharide-induced Injury of Renal Tubular Epithelial Cells by Blocking Janus Kinase 2/Signal Transducer and Activator of Transcription 3 Signaling
Suo Xu, Jiye Luo, Yanli Wang, Xiaobing Chen
Chinese Journal of Physiology.2024; 67(1): 47. CrossRef - Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications
Zhili Wu, Yanru Zhu, Wenchao Liu, Balamuralikrishnan Balasubramanian, Xiao Xu, Junhu Yao, Xinjian Lei
Antioxidants.2024; 13(3): 352. CrossRef - Mechanisms and regulations of ferroptosis
Xu-Dong Zhang, Zhong-Yuan Liu, Mao-Sen Wang, Yu-Xiang Guo, Xiang-Kun Wang, Kai Luo, Shuai Huang, Ren-Feng Li
Frontiers in Immunology.2023;[Epub] CrossRef - Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases
Yumin Wang, Jing Hu, Shuang Wu, Joshua S. Fleishman, Yulin Li, Yinshi Xu, Wailong Zou, Jinhua Wang, Yukuan Feng, Jichao Chen, Hongquan Wang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef
- Fatty Acid Binding Protein-4 Silencing Inhibits Ferroptosis to Alleviate Lipopolysaccharide-induced Injury of Renal Tubular Epithelial Cells by Blocking Janus Kinase 2/Signal Transducer and Activator of Transcription 3 Signaling

Review Articles
- Miscellaneous
- Brown Adipose Tissue: Activation and Metabolism in Humans
- Imane Hachemi, Mueez U-Din
- Endocrinol Metab. 2023;38(2):214-222. Published online March 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1659
- Funded: Academy of Finland, Turku University Hospital, Finnish Diabetes Research Foundation, Finnish Cultural Foundation, Finnish-Norwegian Medical Research Foundation, Juhani Aho Foundation, Jalmari ja Rauha Ahokkaan Säätiö
- 5,989 View
- 445 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
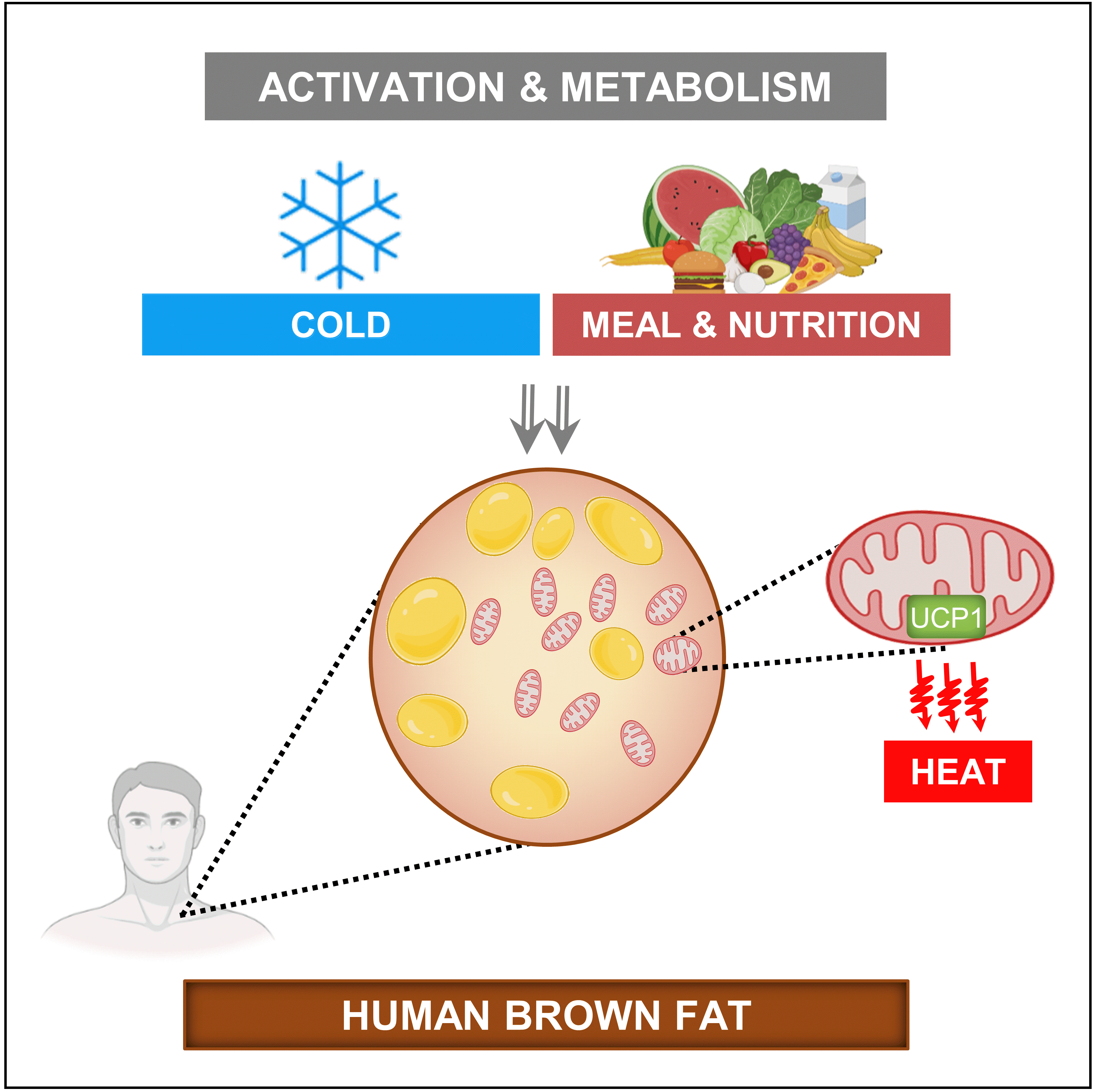
ePub - Brown adipose tissue (BAT) is a thermogenic organ contributing to non-shivering thermogenesis. BAT becomes active under cold stress via sympathetic nervous system activation. However, recent evidence has suggested that BAT may also be active at thermoneutrality and in a postprandial state. BAT has superior energy dissipation capacity compared to white adipose tissue (WAT) and muscles. Thus, it has been proposed that the recruitment and activation of additional BAT may increase the overall energy-expending capacity in humans, potentially improving current whole-body weight management strategies. Nutrition plays a central role in obesity and weight management. Thus, this review discusses human studies describing BAT hyper-metabolism after dietary interventions. Nutritional agents that can potentially recruit brown adipocytes via the process of BAT-WAT transdifferentiation are also discussed.
-
Citations
Citations to this article as recorded by- Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21
Yinhua Ni, Liujie Zheng, Liqian Zhang, Jiamin Li, Yuxiang Pan, Haimei Du, Zhaorong Wang, Zhengwei Fu
The Journal of Nutritional Biochemistry.2024; 125: 109569. CrossRef - A natural sustained-intestinal release formulation of red chili pepper extracted capsaicinoids (Capsifen®) safely modulates energy balance and endurance performance: a randomized, double-blind, placebo-controlled study
N. Roopashree, Das S. Syam, I. M. Krishnakumar, K. N. Mala, Bradley S. Fleenor, Jestin Thomas
Frontiers in Nutrition.2024;[Epub] CrossRef - MRI Methods to Visualize and Quantify Adipose Tissue in Health and Disease
Katerina Nikiforaki, Kostas Marias
Biomedicines.2023; 11(12): 3179. CrossRef
- Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21

- Thyroid
- The Physiological Functions and Polymorphisms of Type II Deiodinase
- Yan Deng, Yi Han, Sheng Gao, Wei Dong, Yang Yu
- Endocrinol Metab. 2023;38(2):190-202. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1599
- Funded: National Natural Science Foundation of China, Fund of the Key Laboratory of Medical Electrophysiology in 2021, China Postdoctoral Science Foundation

- 4,298 View
- 174 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Type II deiodinase (DIO2) is thought to provide triiodothyronine (T3) to the nucleus to meet intracellular needs by deiodinating the prohormone thyroxine. DIO2 is expressed widely in many tissues and plays an important role in a variety of physiological processes, such as controlling T3 content in developing tissues (e.g., bone, muscles, and skin) and the adult brain, and regulating adaptive thermogenesis in brown adipose tissue (BAT). However, the identification and cloning of DIO2 have been challenging. In recent years, several clinical investigations have focused on the Thr92Ala polymorphism, which is closely correlated with clinical syndromes such as type 2 diabetes, obesity, hypertension, and osteoarthritis. Thr92Ala-DIO2 was also found to be related to bone and neurodegenerative diseases and tumors. However, relatively few reviews have synthesized research on individual deiodinases, especially DIO2, in the past 5 years. This review summarizes current knowledge regarding the physiological functions of DIO2 in thyroid hormone signaling and adaptive thermogenesis in BAT and the brain, as well as the associations between Thr92Ala-DIO2 and bone and neurodegenerative diseases and tumors. This discussion is expected to provide insights into the physiological functions of DIO2 and the clinical syndromes associated with Thr92Ala-DIO2.
-
Citations
Citations to this article as recorded by- Noncatalytic Reductive Deiodination of Thyroid Hormones. Electrochemistry and Quantum Chemical Calculations
Piotr P. Romańczyk, Stefan S. Kurek
ChemElectroChem.2024;[Epub] CrossRef
- Noncatalytic Reductive Deiodination of Thyroid Hormones. Electrochemistry and Quantum Chemical Calculations

Letter
- Thyroid
- Frequency of TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients (Endocrinol Metab 2022;37:652-63, Heera Yang et al.)
- Sue Youn Kim, Chan Kwon Jung
- Endocrinol Metab. 2022;37(6):947-948. Published online November 10, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1596
- Funded: National Research Foundation of Korea, Ministry of Science and ICT

- 1,674 View
- 158 Download
- 1 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung
International Journal of Thyroidology.2023; 16(1): 1. CrossRef
- 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules

Original Articles
- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Metformin and Cervical Cancer Risk in Patients with Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea
- Hyun Min Kim, Min Jin Kang, Sun Ok Song
- Endocrinol Metab. 2022;37(6):929-937. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1613
- Correction in: Endocrinol Metab 2023;38(1):174
- Funded: National Health Insurance Ilsan Hospital
- 2,439 View
- 218 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
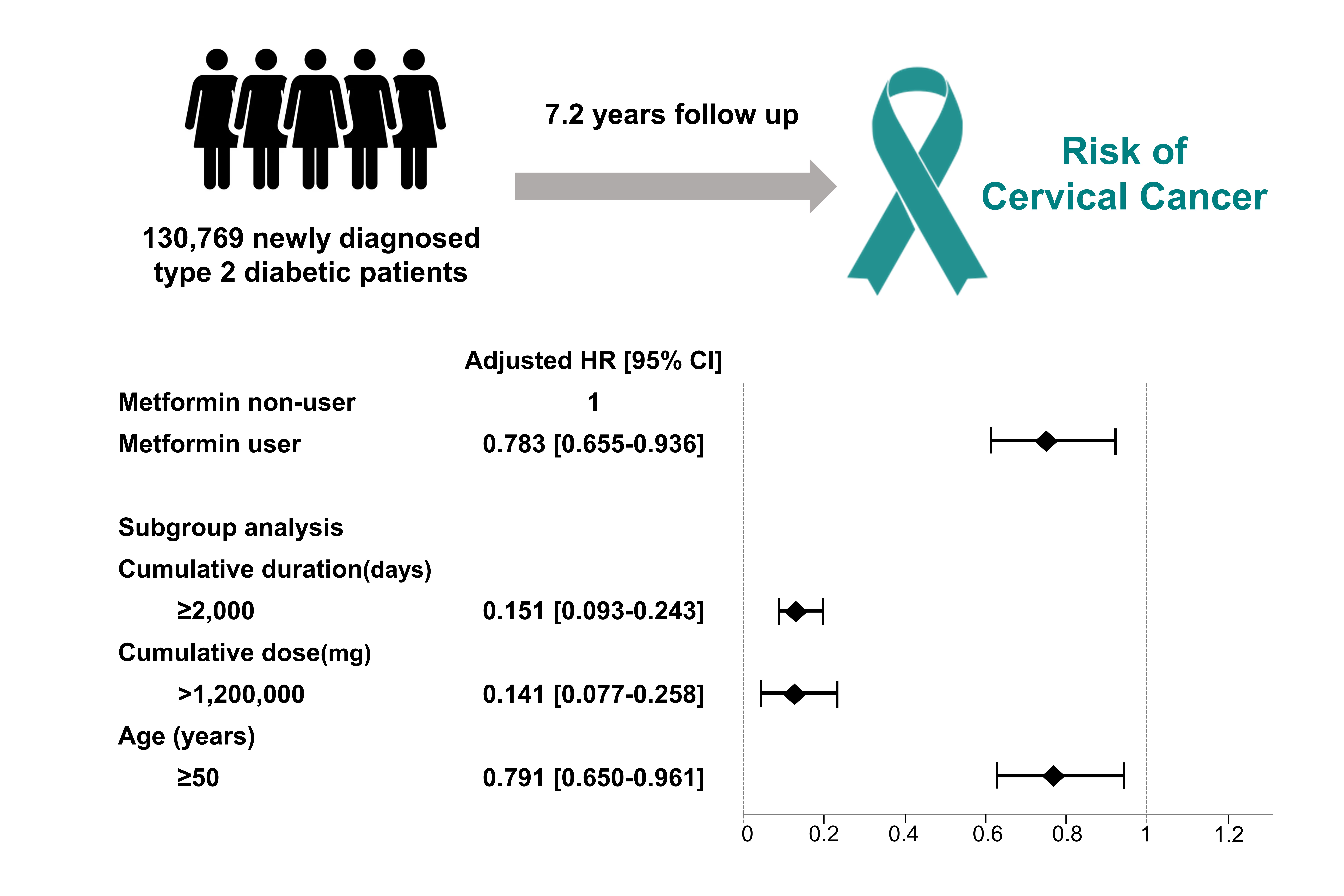
ePub - Background
Cervical cancer is a prevalent malignancy that is a major health problem for women worldwide. The cancer-preventive properties of metformin are well-known, but insufficient data have been reported regarding its relationship to cervical cancer. Therefore, in a nationwide population-based study, we investigated the association between metformin use and cervical cancer incidence in patients with newly diagnosed type 2 diabetes.
Methods
This retrospective cohort study used the Korean National Health Insurance claims database. Individuals newly diagnosed with type 2 diabetes between January 2005 and December 2009 were included. The occurrence of cervical cancer was explored by matching for age, economic status, region of residence, and use of anti-diabetic medication.
Results
In total, 66,013 metformin users and 64,756 non-users were analyzed. Cervical cancer occurred in 219 metformin users (0.33%) and 274 metformin non-users (0.42%) (hazard ratio [HR], 0.783; 95% confidence interval [CI], 0.655 to 0.036; P=0.007). Moreover, cervical cancer risk was considerably reduced in those treated with a high dose (>1,200,000 mg) or for an extended period (≥2,000 days) compared to non-users (HR, 0.151; 95% CI, 0.093 to 0.243; P<0.001; and HR, 0.141; 95% CI, 0.077 to 0.258; P<0.001). The incidence was also significantly lower in metformin users among those over 50 years old (HR, 0.791; 95% CI, 0.650 to 0.961; P<0.001).
Conclusion
Metformin use in patients with newly diagnosed diabetes was associated with a lower risk of cervical cancer in Korea. Furthermore, a significant association was found between the use of metformin and cervical cancer in a dose- and duration-dependent manner and among those over 50 years old. -
Citations
Citations to this article as recorded by- Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp
Mohamad Ali Hijazi, André Gessner, Nahed El-Najjar
Cancers.2023; 15(12): 3199. CrossRef - Network-based drug repurposing for HPV-associated cervical cancer
Faheem Ahmed, Young Jin Yang, Anupama Samantasinghar, Young Woo Kim, Jeong Beom Ko, Kyung Hyun Choi
Computational and Structural Biotechnology Journal.2023; 21: 5186. CrossRef - The Use of Metformin and Postoperative Insulin Pump Were Predictive Factors for Outcomes of Diabetic Colorectal Cancer Patients after Surgery
Xu-Rui Liu, Fei Liu, Zi-Wei Li, Quan Lv, Xin-Peng Shu, Lian-Shuo Li, Yue Tong, Wei Zhang, Dong Peng
Nutrition and Cancer.2023; 75(10): 1926. CrossRef
- Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp

- Diabetes, Obesity and Metabolism
- Gemigliptin Alleviates Succinate-Induced Hepatic Stellate Cell Activation by Ameliorating Mitochondrial Dysfunction
- Giang Nguyen, So Young Park, Dinh Vinh Do, Dae-Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2022;37(6):918-928. Published online November 15, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1530
- Funded: National Research Foundation of Korea
- 3,455 View
- 230 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
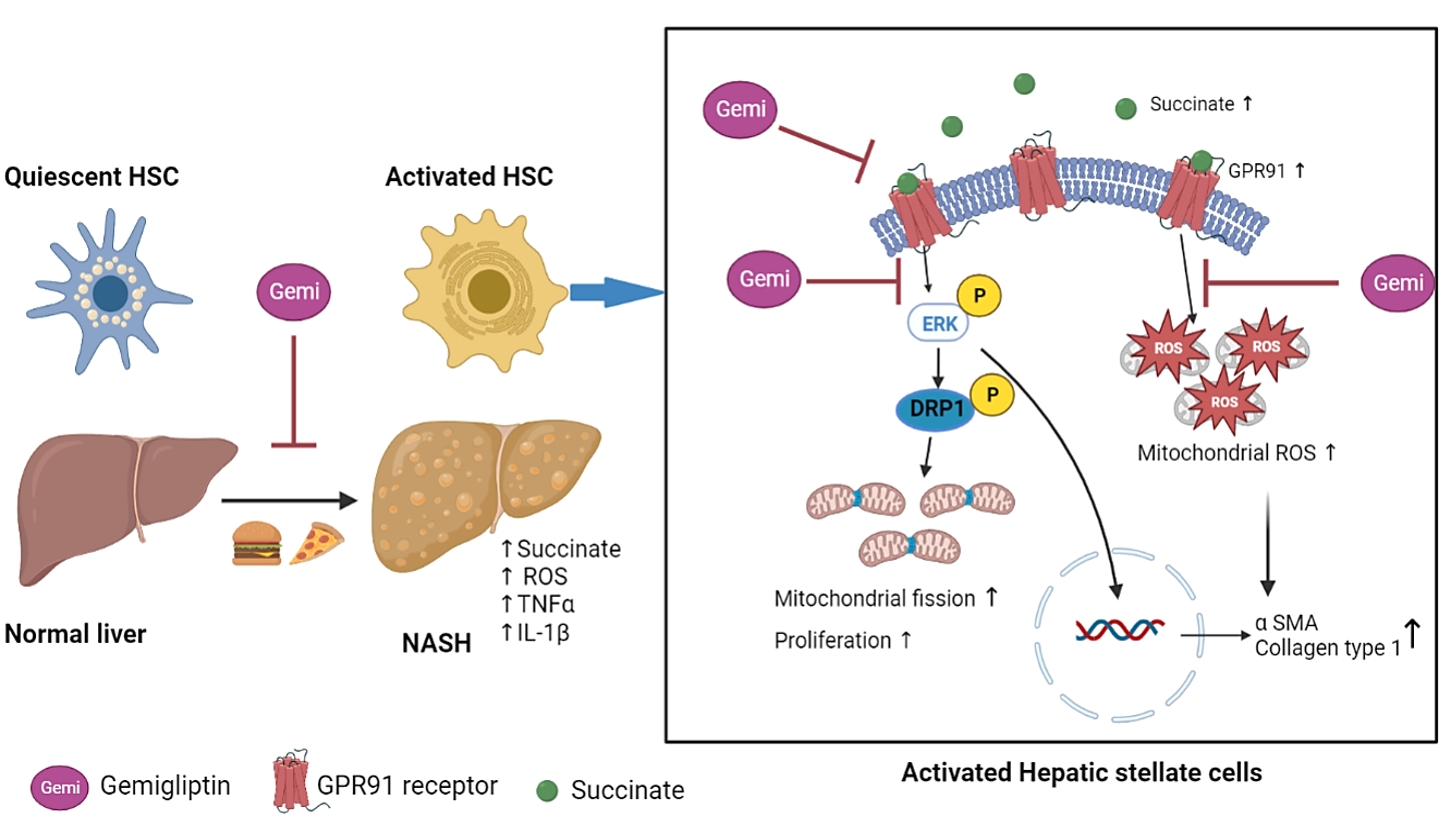
ePub - Background
Dipeptidyl peptidase-4 inhibitors (DPP-4Is) are used clinically as oral antidiabetic agents. Although DPP-4Is are known to ameliorate liver fibrosis, the protective mechanism of DPP-4Is in liver fibrosis remains obscure. In this study, gemigliptin was used to investigate the potential of DPP-4Is to alleviate the progression of liver fibrosis.
Methods
To clarify the effects and mechanisms of gemigliptin, we conducted various experiments in LX-2 cells (immortalized human hepatic stellate cells [HSCs], the principal effectors of hepatic fibrogenesis), which were activated by succinate and exhibited elevated expression of α-smooth muscle actin, collagen type 1, and pro-inflammatory cytokines and increased cell proliferation. In vivo, we examined the effects and mechanisms of gemigliptin on a high-fat, high-cholesterol–induced mouse model of nonalcoholic steatohepatitis (NASH).
Results
Gemigliptin decreased the expression of fibrogenesis markers and reduced the abnormal proliferation of HSCs. In addition, gemigliptin reduced the succinate-induced production of mitochondrial reactive oxygen species (ROS), intracellular ROS, and mitochondrial fission in HSCs. Furthermore, in the mouse model of NASH-induced liver fibrosis, gemigliptin alleviated both liver fibrosis and mitochondrial dysfunction.
Conclusion
Gemigliptin protected against HSC activation and liver fibrosis by alleviating mitochondrial dysfunction and ROS production, indicating its potential as a strategy for preventing the development of liver disease. -
Citations
Citations to this article as recorded by- Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats
Woo Kwon Jung, Su-Bin Park, Hwa Young Yu, Junghyun Kim
Heliyon.2024; 10(8): e29362. CrossRef - Gemigliptin, a DPP4 inhibitor, ameliorates nonalcoholic steatohepatitis through AMP-activated protein kinase-independent and ULK1-mediated autophagy
Youngmi Song, Hyekyung Yang, Juhee Kim, Yoonjin Lee, Sung-Ho Kim, In-Gu Do, Cheol-Young Park
Molecular Metabolism.2023; 78: 101806. CrossRef - DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once?
Ji Cheol Bae
Endocrinology and Metabolism.2022; 37(6): 858. CrossRef
- Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats

- Diabetes, Obesity and Metabolism
- Inhibition of miR-146a-5p and miR-8114 in Insulin-Secreting Cells Contributes to the Protection of Melatonin against Stearic Acid-Induced Cellular Senescence by Targeting Mafa
- Shenghan Su, Qingrui Zhao, Lingfeng Dan, Yuqing Lin, Xuebei Li, Yunjin Zhang, Chunxiao Yang, Yimeng Dong, Xiaohan Li, Romano Regazzi, Changhao Sun, Xia Chu, Huimin Lu
- Endocrinol Metab. 2022;37(6):901-917. Published online December 7, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1565
- Funded: Excellent Youth Foundation of Heilongjiang Province of China, National Natural Science Foundation of China, Harbin Medical University, China Scholarship Council

- 2,408 View
- 220 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Chronic exposure to elevated levels of saturated fatty acids results in pancreatic β-cell senescence. However, targets and effective agents for preventing stearic acid-induced β-cell senescence are still lacking. Although melatonin administration can protect β-cells against lipotoxicity through anti-senescence processes, the precise underlying mechanisms still need to be explored. Therefore, we investigated the anti-senescence effect of melatonin on stearic acid-treated mouse β-cells and elucidated the possible role of microRNAs in this process.
Methods
β-Cell senescence was identified by measuring the expression of senescence-related genes and senescence-associated β-galactosidase staining. Gain- and loss-of-function approaches were used to investigate the involvement of microRNAs in stearic acid-evoked β-cell senescence and dysfunction. Bioinformatics analyses and luciferase reporter activity assays were applied to predict the direct targets of microRNAs.
Results
Long-term exposure to a high concentration of stearic acid-induced senescence and upregulated miR-146a-5p and miR- 8114 expression in both mouse islets and β-TC6 cell lines. Melatonin effectively suppressed this process and reduced the levels of these two miRNAs. A remarkable reversibility of stearic acid-induced β-cell senescence and dysfunction was observed after silencing miR-146a-5p and miR-8114. Moreover, V-maf musculoaponeurotic fibrosarcoma oncogene homolog A (Mafa) was verified as a direct target of miR-146a-5p and miR-8114. Melatonin also significantly ameliorated senescence and dysfunction in miR-146a-5pand miR-8114-transfected β-cells.
Conclusion
These data demonstrate that melatonin protects against stearic acid-induced β-cell senescence by inhibiting miR-146a- 5p and miR-8114 and upregulating Mafa expression. This not only provides novel targets for preventing stearic acid-induced β-cell dysfunction, but also points to melatonin as a promising drug to combat type 2 diabetes progression. -
Citations
Citations to this article as recorded by- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice
Yuxuan Chen, Rou Peng, Yi Qian, Yizhou Lu, Liyao Chen, Meiling Yu, Minjiao Jiang, Wei Wu, Shengfeng Lu
Gene.2024; 897: 148090. CrossRef - MiR-126 and miR-146a as Melatonin-Responsive Biomarkers for Neonatal Brain Ischemia
Maria Cristina Albertini, Tania Vanzolini, Serafina Perrone, Michael D. Weiss, Giuseppe Buonocore, Valentina Dell’Orto, Walter Balduini, Silvia Carloni
Journal of Molecular Neuroscience.2023; 73(9-10): 763. CrossRef
- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice

- Thyroid
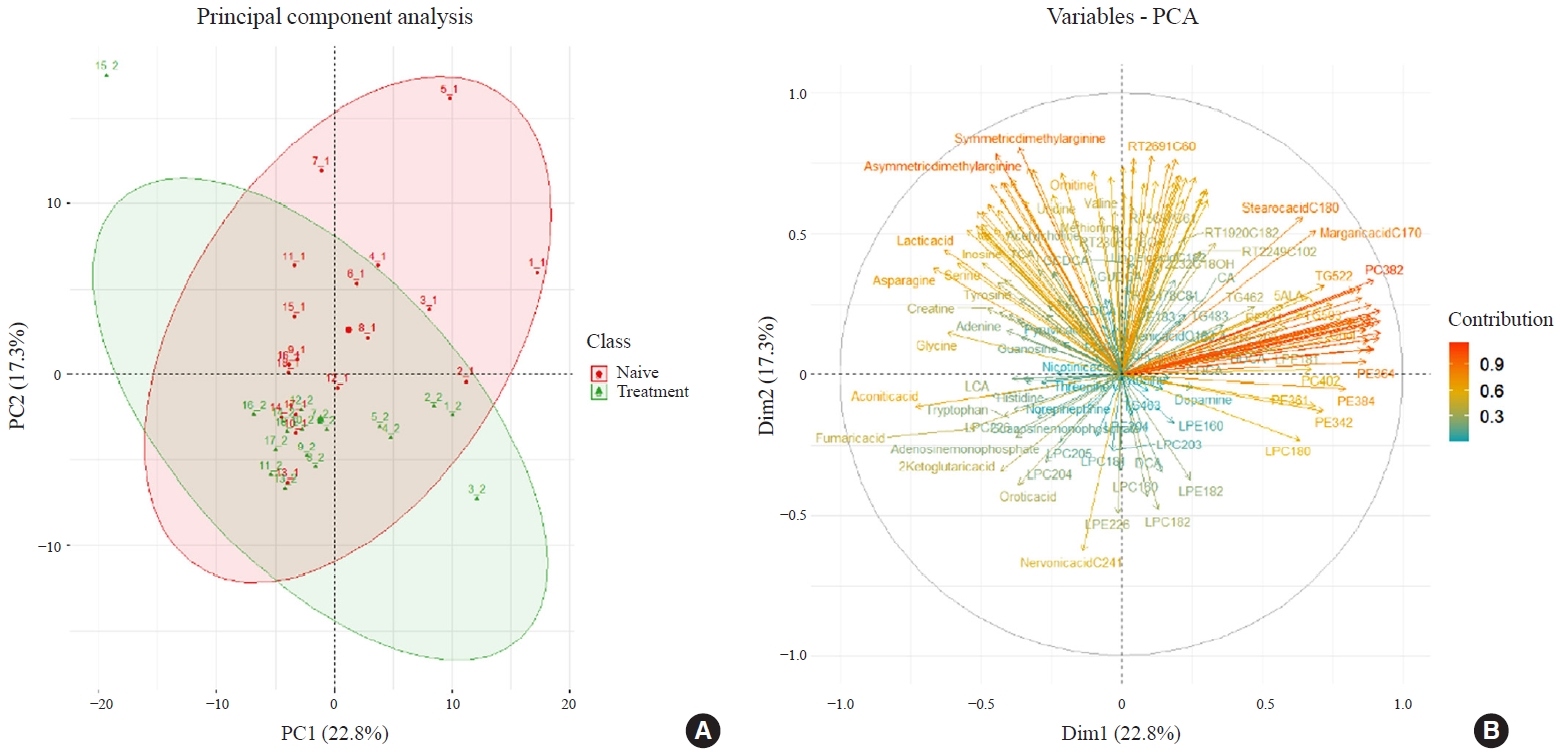
- Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
- Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
- Endocrinol Metab. 2022;37(6):891-900. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1590
- Funded: National Research Foundation of Korea, Ministry of Science, ICT and Future Planning, Korea Health Industry Development Institute, Ministry of Health and Welfare, Chungnam National University Hospital, Chungnam National University
- 2,497 View
- 255 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
An excess of thyroid hormones in Graves’ disease (GD) has profound effects on systemic energy metabolism that are currently partially understood. In this study, we aimed to provide a comprehensive understanding of the metabolite changes that occur when patients with GD transition from hyperthyroidism to euthyroidism with methimazole treatment.
Methods
Eighteen patients (mean age, 38.6±14.7 years; 66.7% female) with newly diagnosed or relapsed GD attending the endocrinology outpatient clinics in a single institution were recruited between January 2019 and July 2020. All subjects were treated with methimazole to achieve euthyroidism. We explored metabolomics by performing liquid chromatography-mass spectrometry analysis of plasma samples of these patients and then performed multivariate statistical analysis of the metabolomics data.
Results
Two hundred metabolites were measured before and after 12 weeks of methimazole treatment in patients with GD. The levels of 61 metabolites, including palmitic acid (C16:0) and oleic acid (C18:1), were elevated in methimazole-naïve patients with GD, and these levels were decreased by methimazole treatment. The levels of another 15 metabolites, including glycine and creatinine, were increased after recovery of euthyroidism upon methimazole treatment in patients with GD. Pathway analysis of metabolomics data showed that hyperthyroidism was closely related to aminoacyl-transfer ribonucleic acid biosynthesis and branched-chain amino acid biosynthesis pathways.
Conclusion
In this study, significant variations of plasma metabolomic patterns that occur during the transition from hyperthyroidism to euthyroidism were detected in patients with GD via untargeted metabolomics analysis. -
Citations
Citations to this article as recorded by- Associations of serum keratin 1 with thyroid function and immunity in Graves’ disease
Chao-Wen Cheng, Wen-Fang Fang, Jiunn-Diann Lin, Appuwawadu Mestri Nipun Lakshitha de Silva
PLOS ONE.2023; 18(11): e0289345. CrossRef
- Associations of serum keratin 1 with thyroid function and immunity in Graves’ disease

- Thyroid
- BRAFV600E Mutation Enhances Estrogen-Induced Metastatic Potential of Thyroid Cancer by Regulating the Expression of Estrogen Receptors
- Minjun Kim, Su-jin Kim, Seong Yun Ha, Zhen Xu, Youngjin Han, Hyeon-Gun Jee, Sun Wook Cho, Young Joo Park, Kyu Eun Lee
- Endocrinol Metab. 2022;37(6):879-890. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1563
- Funded: National Research Foundation of Korea, Ministry of Science, ICT and Future Planning, Seoul National University Hospital, American Thyroid Association, Korean Cancer Association

- 2,780 View
- 215 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Cross-talk between mitogen-activated protein kinase and estrogen has been reported; however, the role of BRAFV600E in the estrogen responsiveness of thyroid cancer is unknown. We elucidated the effect of BRAFV600E on the estrogen-induced increase in metastatic potential in thyroid cancer.
Methods
Using a pair of cell lines, human thyroid cell lines which harbor wild type BRAF gene (Nthy/WT) and Nthy/BRAFV600E (Nthy/V600E), the expression of estrogen receptors (ERs) and estrogen-induced metastatic phenotypes were evaluated. Susceptibility to ERα- and ERβ-selective agents was evaluated to confirm differential ER expression. ESR expression was analyzed according to BRAFV600E status and age (≤50 years vs. >50 years) using The Cancer Genome Atlas (TCGA) data.
Results
Estradiol increased the ERα/ERβ expression ratio in Nthy/V600E, whereas the decreased ERα/ERβ expression ratio was found in Nthy/WT. BRAFV600E-mutated cell lines showed a higher E2-induced increase in metastatic potential, including migration, invasion, and anchorage-independent growth compared with Nthy/WT. An ERα antagonist significantly inhibited migration in Nthy/V600E cells, whereas an ERβ agonist was more effective in Nthy/WT. In the BRAFV600E group, ESR1/ESR2 ratio was significantly higher in younger age group (≤50 years) compared with older age group (>50 years) by TCGA data analysis.
Conclusion
Our data show that BRAFV600E mutation plays a crucial role in the estrogen responsiveness of thyroid cancer by regulating ER expression. Therefore, BRAFV600E might be used as a biomarker when deciding future hormone therapies based on estrogen signaling in thyroid cancer patients. -
Citations
Citations to this article as recorded by- The importance of protein domain mutations in cancer therapy
Kiran Kumar Chitluri, Isaac Arnold Emerson
Heliyon.2024; 10(6): e27655. CrossRef - Three cases of thyroid cancer in transgender female veterans receiving gender-affirming estrogen treatment
John D. Christensen, Hiba T. Basheer
Endocrine and Metabolic Science.2024; 15: 100177. CrossRef - Thyroid Cancer Prevalence, Risk Exposure, and Clinical Features Among Transgender Female Veterans
John David Christensen, Hiba T Basheer, Jose Joaquin Lado Abeal
Journal of the Endocrine Society.2024;[Epub] CrossRef - Association of DNA Promoter Methylation and BRAF Mutation in Thyroid Cancer
Farzana Jasmine, Briseis Aschebrook-Kilfoy, Mohammad M. Rahman, Garrett Zaagman, Raymon H. Grogan, Mohammed Kamal, Habibul Ahsan, Muhammad G. Kibriya
Current Oncology.2023; 30(3): 2978. CrossRef - Editorial: Recent advances in papillary thyroid carcinoma: Progression, treatment and survival predictors
Erivelto Martinho Volpi, Margarita Carmen Ramirez-Ortega, Jose Federico Carrillo
Frontiers in Endocrinology.2023;[Epub] CrossRef
- The importance of protein domain mutations in cancer therapy

Review Article
- Thyroid
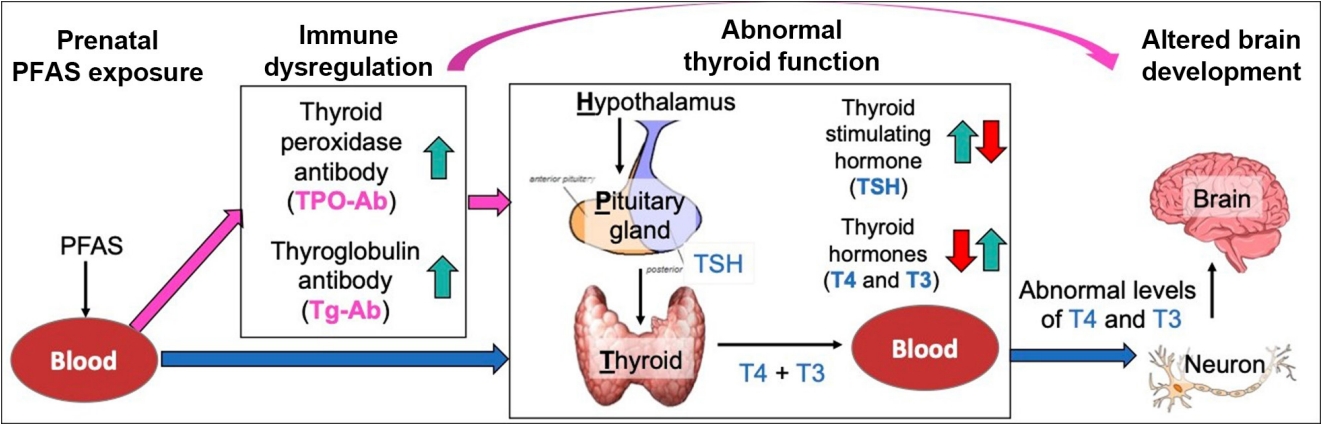
- Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Maternal Thyroid Dysfunction, and Child Autism Spectrum Disorder
- Hyeong-Moo Shin, Jiwon Oh, Rebecca J. Schmidt, Elizabeth N. Pearce
- Endocrinol Metab. 2022;37(6):819-829. Published online November 23, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1598
- Funded: National Institute of Environmental Health Sciences
- 6,336 View
- 131 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Autism spectrum disorder (ASD), with its high economic and societal costs, is a growing public health concern whose prevalence has risen steadily over the last two decades. Although actual increased incidence versus improved diagnosis remains controversial, the increased prevalence of ASD suggests non-inherited factors as likely contributors. There is increasing epidemiologic evidence that abnormal maternal thyroid function during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders. Prenatal exposure to endocrine-disrupting chemicals such as per- and polyfluoroalkyl substances (PFAS) is known to disrupt thyroid function and can affect early brain development; thus, thyroid dysfunction is hypothesized to mediate this relationship. The concept of a potential pathway from prenatal PFAS exposure through thyroid dysfunction to ASD etiology is not new; however, the extant literature on this topic is scant. The aim of this review is to evaluate and summarize reports with regard to potential mechanisms in this pathway.
-
Citations
Citations to this article as recorded by- Endocrine Disruptors and Thyroid Health
Elizabeth N. Pearce
Endocrine Practice.2024; 30(2): 172. CrossRef - Maternal Thyroid Dysfunction During Pregnancy as an Etiologic Factor in Autism Spectrum Disorder: Challenges and Opportunities for Research
Zoe B. Kaplan, Elizabeth N. Pearce, Sun Y. Lee, Hyeong-Moo Shin, Rebecca J. Schmidt
Thyroid®.2024; 34(2): 144. CrossRef - Effects of Endocrine-Disrupting Chemicals on Human Health
Jun Hyung Lee, Sung-Eun Cho
Laboratory Medicine Online.2023; 13(3): 129. CrossRef
- Endocrine Disruptors and Thyroid Health


 KES
KES

 First
First Prev
Prev



