Search
- Page Path
- HOME > Search
- Calcium & bone metabolism
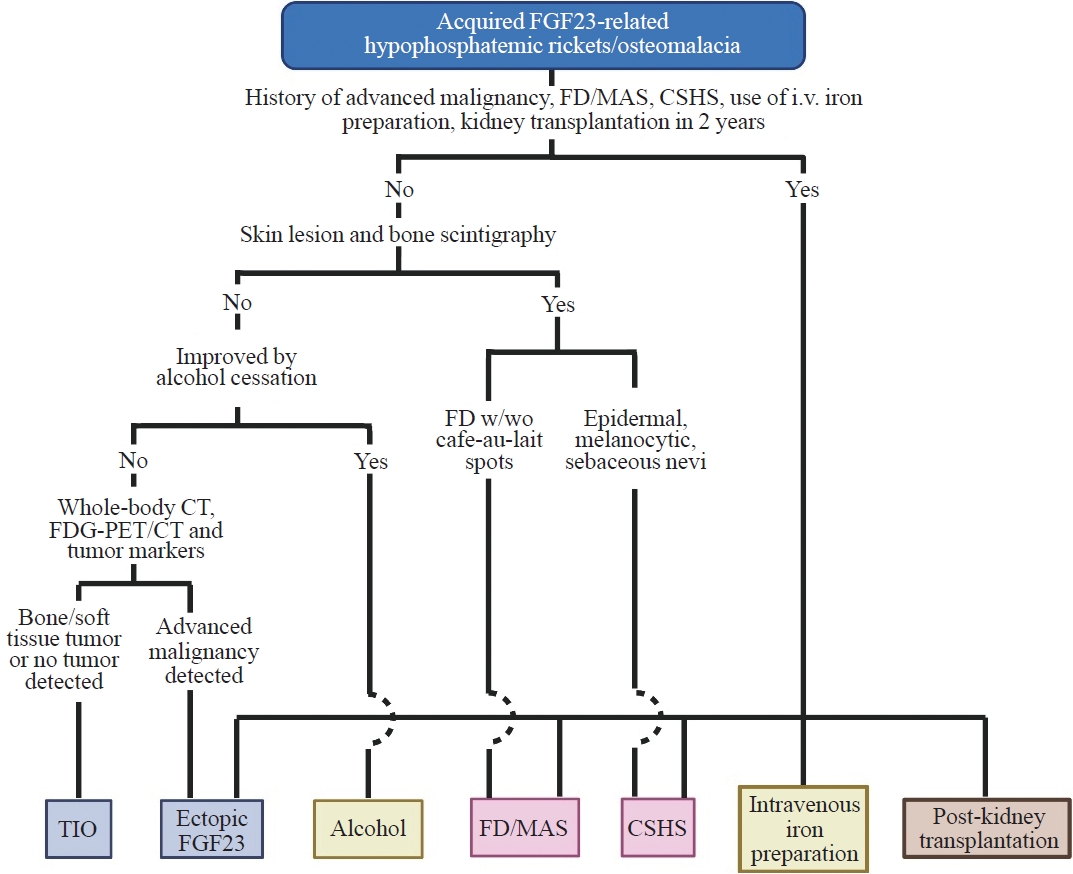
- Acquired Forms of Fibroblast Growth Factor 23-Related Hypophosphatemic Osteomalacia
- Nobuaki Ito, Naoko Hidaka, Hajime Kato
- Endocrinol Metab. 2024;39(2):255-261. Published online March 11, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1908
- 1,359 View
- 73 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Fibroblast growth factor 23 (FGF23) is a pivotal humoral factor for the regulation of serum phosphate levels and was first identified in patients with autosomal dominant hypophosphatemic rickets and tumor-induced osteomalacia (TIO), the most common form of acquired FGF23-related hypophosphatemic rickets/osteomalacia (FGF23rHR). After the identification of FGF23, many other inherited and acquired forms of FGF23rHR were reported. In this review article, the detailed features of each acquired FGF23rHR are discussed, including TIO, ectopic FGF23 syndrome with malignancy, fibrous dysplasia/McCune-Albright syndrome, Schimmelpenning-Feuerstein-Mims syndrome/cutaneous skeletal hypophosphatemia syndrome, intravenous iron preparation-induced FGF23rHR, alcohol consumption-induced FGF23rHR, and post-kidney transplantation hypophosphatemia. Then, an approach for the differential diagnosis and therapeutic options for each disorder are concisely introduced. Currently, the majority of endocrinologists might only consider TIO when encountering patients with acquired FGF23rHR; an adequate differential diagnosis can reduce medical costs and invasive procedures such as positron emission tomography/computed tomography and venous sampling to identify FGF23-producing tumors. Furthermore, some acquired FGF23rHRs, such as intravenous iron preparation/alcohol consumption-induced FGF23rHR, require only cessation of drugs or alcohol to achieve full recovery from osteomalacia.

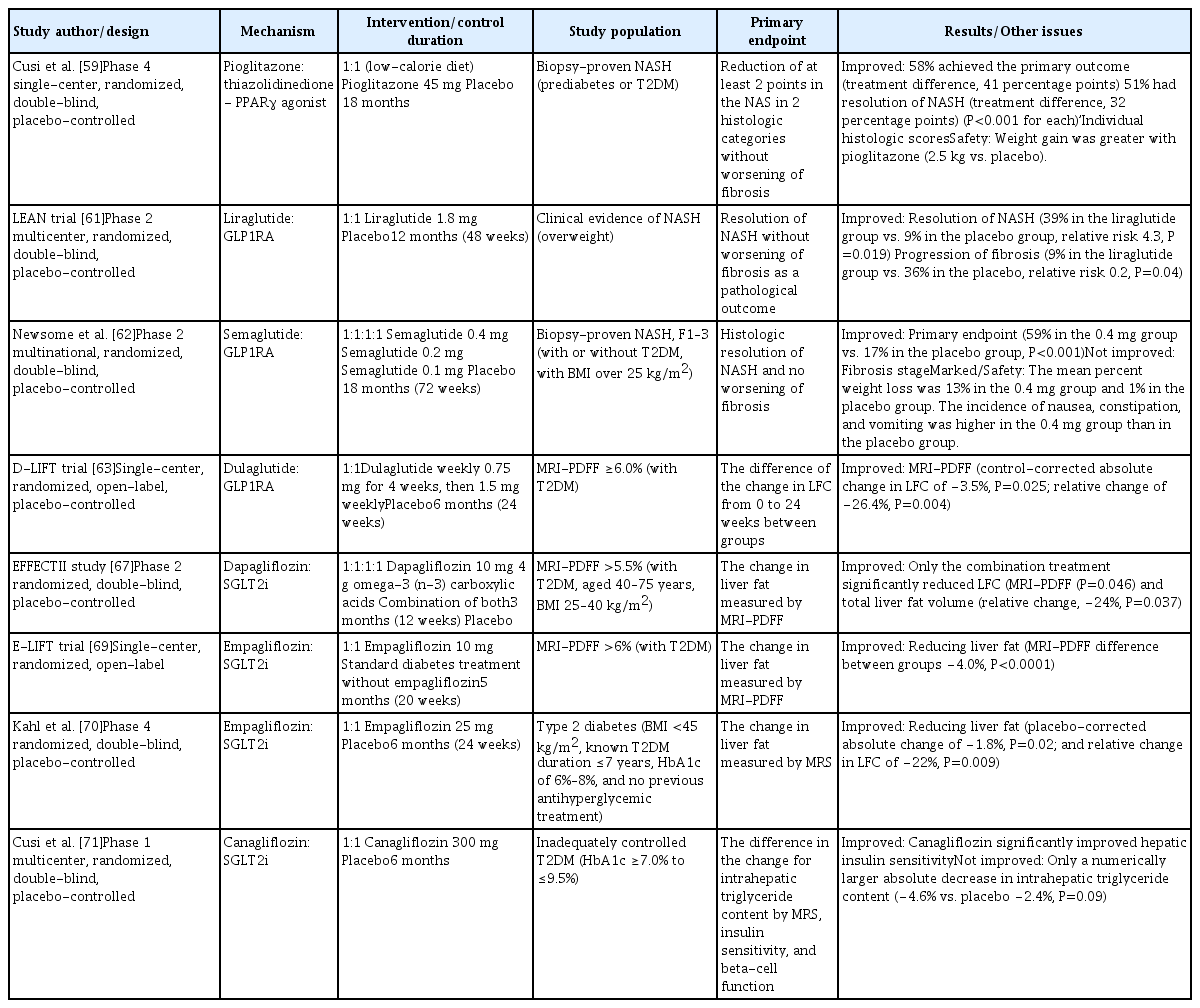
- Diabetes, obesity and metabolism
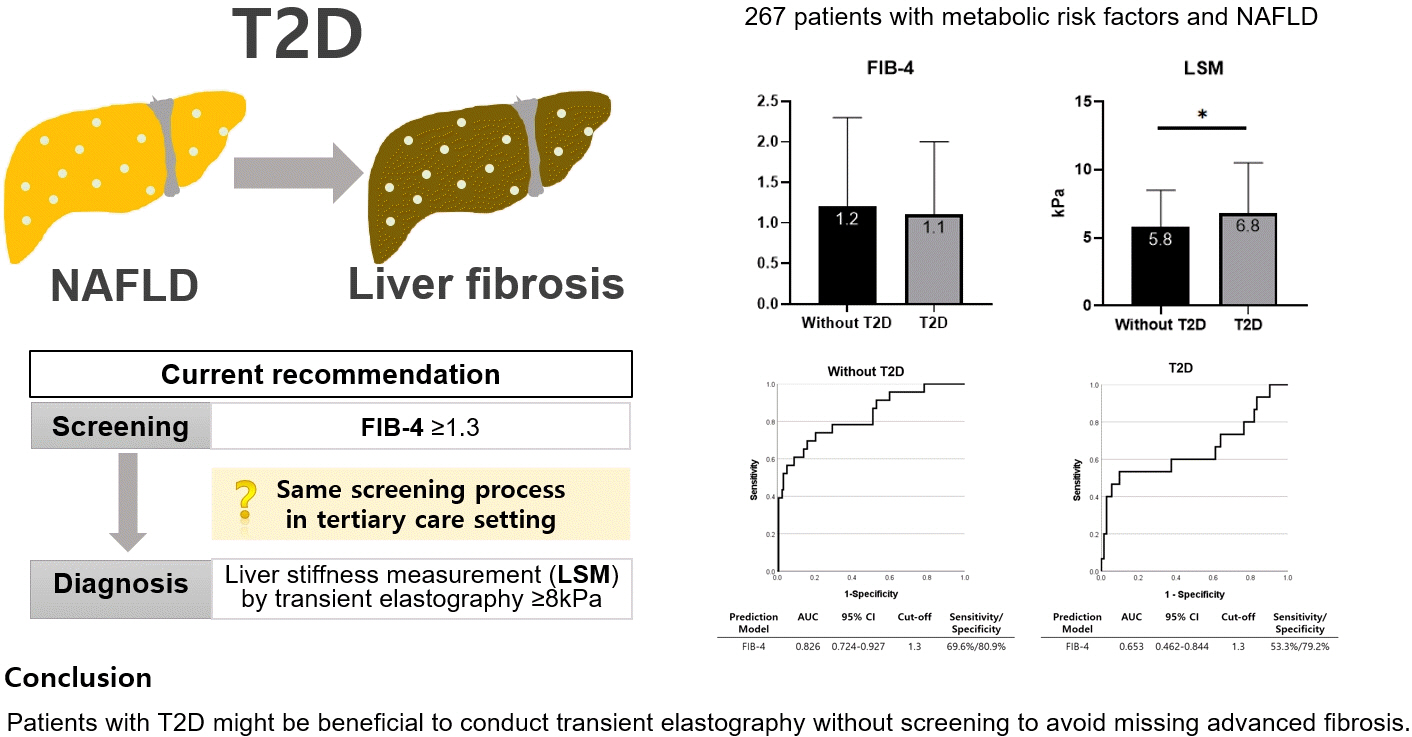
- Performance of Simple Fibrosis Score in Non-Alcoholic Fatty Liver Disease with and without Type 2 Diabetes
- Seung Min Chung, Min Kyu Kang, Jun Sung Moon, Jung Gil Park
- Endocrinol Metab. 2023;38(2):277-281. Published online March 13, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1635
- 1,852 View
- 97 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - This cross-sectional study enrolled 267 patients with metabolic risk factors and established non-alcoholic fatty liver disease in the prospective cohort. The performance of fibrosis-4 (FIB-4) score (≥1.3) to diagnose advanced fibrosis using transient elastography (liver stiffness measurement [LSM] ≥8 kPa) was analyzed. Comparing patients with type 2 diabetes (T2D, n=87) and without (n=180), not FIB-4, but LSM was significantly higher in T2D (P=0.026). The prevalence of advanced fibrosis was 17.2% in T2D and 12.8% in non-T2D. FIB-4 exhibited higher proportion of false negatives in T2D patients (10.9%) than those without (5.2%). The diagnostic performance of FIB-4 was suboptimal in T2D (area under curve [AUC], 0.653; 95% confidence interval [CI], 0.462 to 0.844) compared to that in non-T2D (AUC, 0.826; 95% CI, 0.724 to 0.927). In conclusion, patients with T2D might be beneficial to conduct transient elastography without screening to avoid missing advanced fibrosis.
-
Citations
Citations to this article as recorded by- Prevalence of High and Moderate Risk of Liver Fibrosis Among Patients With Diabetes at a Noncommunicable Diseases (NCD) Clinic in a Primary Healthcare Center in Northern India
Anubhav Mondal, Aninda Debnath, Ghurumourthy Dhandapani, Abhishek Sharma, Shveta Lukhmana, Geeta Yadav
Cureus.2023;[Epub] CrossRef
- Prevalence of High and Moderate Risk of Liver Fibrosis Among Patients With Diabetes at a Noncommunicable Diseases (NCD) Clinic in a Primary Healthcare Center in Northern India

- Diabetes, Obesity and Metabolism
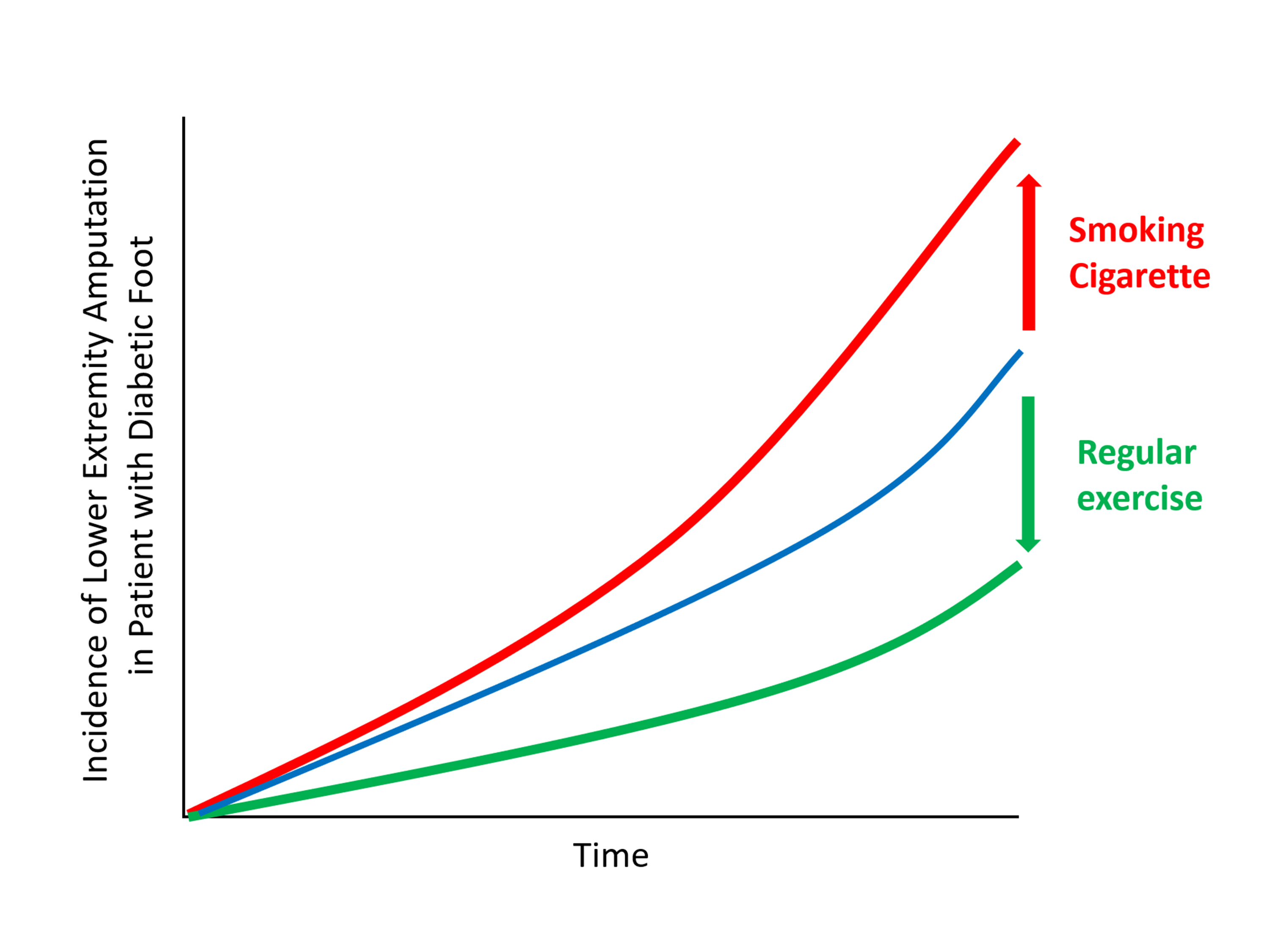
Big Data Articles (National Health Insurance Service Database) - Association among Current Smoking, Alcohol Consumption, Regular Exercise, and Lower Extremity Amputation in Patients with Diabetic Foot: Nationwide Population-Based Study
- Yoon Jae Lee, Kyung-Do Han, Jun Hyeok Kim
- Endocrinol Metab. 2022;37(5):770-780. Published online October 12, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1519
- 3,339 View
- 203 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The present study investigates whether modifiable behavioral factors of current cigarette smoking, heavy alcohol consumption, and regular exercise are associated with risk of lower extremity amputation (LEA) in diabetic patients.
Methods
A total of 2,644,440 diabetic patients (aged ≥20 years) was analyzed using the database of the Korean National Health Insurance Service. Cox proportional hazard regression was used to assess adjusted hazard ratios (HRs) for the behavioral factors with risk of LEA under adjustment for potential confounders.
Results
The risk of LEA was significantly increased by current cigarette smoking and heavy alcohol consumption (HR, 1.436; 95% confidence interval [CI], 1.367 to 1.508 and HR, 1.082; 95% CI, 1.011 to 1.158) but significantly decreased with regular exercise (HR, 0.745; 95% CI, 0.706 to 0.786) after adjusting for age, sex, smoking, alcohol consumption, exercise, low income, hypertension, dyslipidemia, body mass index, using insulin or oral antidiabetic drugs, and diabetic duration. A synergistically increased risk of LEA was observed with larger number of risky behaviors.
Conclusion
Modification of behaviors of current smoking, heavy alcohol intake, and exercise prevents LEA and can improve physical, emotional, and social quality of life in diabetic patients. -
Citations
Citations to this article as recorded by- Adjuvant effect of antimicrobial photodynamic therapy (aPDT) in the treatment of diabetic foot ulcers: A case series
Rita de Cassia Ferreira, Rebeca Boltes Cecatto, Silvana Torres Perez, Raquel Agnelli Mesquita‐Ferrari, Sandra Kalil Bussadori, Cinthya Cosme Duran, Anna Carolina Tempestini Horliana, Kristianne Porta Santos Fernandes
Journal of Biophotonics.2024;[Epub] CrossRef - Factors associated with diabetic foot ulcers and lower limb amputations in type 1 and type 2 diabetes supported by real‐world data from the German/Austrian DPV registry
Alexander J. Eckert, Stefan Zimny, Marcus Altmeier, Ana Dugic, Anton Gillessen, Latife Bozkurt, Gabriele Götz, Wolfram Karges, Frank J. Wosch, Stephan Kress, Reinhard W. Holl
Journal of Diabetes.2024;[Epub] CrossRef - Investigating Diabetic Foot Pathophysiology and Amputation Prevention Strategies through Behavioral Modification
Jun Hyeok Kim
Journal of Wound Management and Research.2023; 19(3): 167. CrossRef
- Adjuvant effect of antimicrobial photodynamic therapy (aPDT) in the treatment of diabetic foot ulcers: A case series

- Diabetes, Obesity and Metabolism
- The Impact of Insulin Resistance on Hepatic Fibrosis among United States Adults with Non-Alcoholic Fatty Liver Disease: NHANES 2017 to 2018
- Ji Cheol Bae, Lauren A. Beste, Kristina M. Utzschneider
- Endocrinol Metab. 2022;37(3):455-465. Published online June 21, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1434
- 4,240 View
- 136 Download
- 9 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to investigate the association of hepatic steatosis with liver fibrosis and to assess the interactive effects of hepatic steatosis and insulin resistance on liver fibrosis in a nationally representative sample of United States adults.
Methods
We conducted a cross-sectional analysis using data from National Health and Nutrition Examination Survey 2017 to 2018, which for the first time included transient elastography to assess liver stiffness and hepatic steatosis. We evaluated the association between hepatic steatosis (using controlled attenuation parameter [CAP]) and clinically significant liver fibrosis (defined as liver stiffness ≥7.5 kPa) using logistic regression with an interaction term for hepatic steatosis and insulin resistance (defined as homeostatic model assessment of insulin resistance ≥3.0).
Results
Among adults undergoing transient elastography (n=2,023), 45.9% had moderate or greater hepatic steatosis and 11.3% had clinically significant liver fibrosis. After adjustment for demographic and metabolic factors, the odds of significant liver fibrosis increased as CAP score rose (odds ratio, 1.35 per standard deviation increment; 95% confidence interval, 1.11 to 1.64). We detected a significant interaction effect between CAP score and insulin resistance on the probability of significant liver fibrosis (P=0.016 for interaction). The probability of significant liver fibrosis increased in the presence of insulin resistance with increasing CAP score, while those without insulin resistance had low probability of significant liver fibrosis, even with high CAP scores.
Conclusion
Individuals with hepatic steatosis had higher odds of fibrosis when insulin resistance was present. Our findings emphasize the importance of the metabolic aspects of the disease on fibrosis risk and suggest a need to better identify patients with metabolic associated fatty liver disease. -
Citations
Citations to this article as recorded by- Association of insulin resistance indicators with hepatic steatosis and fibrosis in patients with metabolic syndrome
Tzu-chia Kuo, Yang-bor Lu, Chieh-lun Yang, Bin Wang, Lin-xin Chen, Ching-ping Su
BMC Gastroenterology.2024;[Epub] CrossRef - No More NAFLD: The Term Is Now MASLD
Ji Cheol Bae
Endocrinology and Metabolism.2024; 39(1): 92. CrossRef - Insulin Resistance/Sensitivity Measures as Screening Indicators of Metabolic-Associated Fatty Liver Disease and Liver Fibrosis
Mohammad E. Khamseh, Mojtaba Malek, Soodeh Jahangiri, Sohrab Nobarani, Azita Hekmatdoost, Marieh Salavatizadeh, Samira Soltanieh, Haleh Chehrehgosha, Hoda Taheri, Zeinab Montazeri, Fereshteh Attaran, Faramarz Ismail-Beigi, Fariba Alaei-Shahmiri
Digestive Diseases and Sciences.2024;[Epub] CrossRef - The association of Neuromedin U levels and non-alcoholic fatty liver disease: A comparative analysis
Murat Keskin, Sercan Avul, Aylin Beyaz, Nizameddin Koca
Heliyon.2024; 10(5): e27291. CrossRef - Oral Insulin Alleviates Liver Fibrosis and Reduces Liver Steatosis in Patients With Metabolic Dysfunction-associated Steatohepatitis and Type 2 Diabetes: Results of Phase II Randomized, Placebo-controlled Feasibility Clinical Trial
Yuval Ishay, Joel Neutel, Yotam Kolben, Ram Gelman, Orly Sneh Arbib, Oliver Lopez, Helena Katchman, Rizwana Mohseni, Miriam Kidron, Yaron Ilan
Gastro Hep Advances.2024; 3(3): 417. CrossRef - Comparative and Predictive Significance of Serum Leptin Levels in Non-alcoholic Fatty Liver Disease
Mehwish Qamar, Abeer Fatima, Ambreen Tauseef, Muhammad I Yousufzai, Ibrahim Liaqat, Qanbar Naqvi
Cureus.2024;[Epub] CrossRef - Greater Severity of Steatosis Is Associated with a Higher Risk of Incident Diabetes: A Retrospective Longitudinal Study
Ji Min Han, Jung Hwan Cho, Hye In Kim, Sunghwan Suh, Yu-Ji Lee, Jung Won Lee, Kwang Min Kim, Ji Cheol Bae
Endocrinology and Metabolism.2023; 38(4): 418. CrossRef - Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis
Byeong Chang Sim, Yea Eun Kang, Sun Kyoung You, Seong Eun Lee, Ha Thi Nga, Ho Yeop Lee, Thi Linh Nguyen, Ji Sun Moon, Jingwen Tian, Hyo Ju Jang, Jeong Eun Lee, Hyon-Seung Yi
Cell Death & Disease.2023;[Epub] CrossRef - Familial clustering of nonalcoholic fatty liver disease in first‐degree relatives of adults with lean nonalcoholic fatty liver disease
Sorachat Niltwat, Chanin Limwongse, Natthinee Charatcharoenwitthaya, Duangkamon Bunditvorapoom, Wimolrak Bandidniyamanon, Phunchai Charatcharoenwitthaya
Liver International.2023; 43(12): 2713. CrossRef - Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease
Jun-Hyuk Lee, Yu-Jin Kwon, Kyongmin Park, Hye Sun Lee, Hoon-Ki Park, Jee Hye Han, Sang Bong Ahn
Nutrients.2022; 14(15): 3039. CrossRef - DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once?
Ji Cheol Bae
Endocrinology and Metabolism.2022; 37(6): 858. CrossRef
- Association of insulin resistance indicators with hepatic steatosis and fibrosis in patients with metabolic syndrome

- Diabetes, Obesity and Metabolism
- State-of-the-Art Overview of the Pharmacological Treatment of Non-Alcoholic Steatohepatitis
- Yongin Cho, Yong-ho Lee
- Endocrinol Metab. 2022;37(1):38-52. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.102
- 4,305 View
- 285 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide, and non-alcoholic steatohepatitis (NASH), a subtype of NAFLD, can progress to cirrhosis, hepatocellular carcinoma, and death. Nevertheless, the current treatment for NAFLD/NASH is limited to lifestyle modifications, and no drugs are currently officially approved as treatments for NASH. Many global pharmaceutical companies are pursuing the development of medications for the treatment of NASH, and results from phase 2 and 3 clinical trials have been published in recent years. Here, we review data from these recent clinical trials and reports on the efficacy of newly developed antidiabetic drugs in NASH treatment.
-
Citations
Citations to this article as recorded by- Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Soyeon Shin, Jaeyoung Kim, Ju Yeon Lee, Jun Kim, Chang-Myung Oh
Journal of Obesity & Metabolic Syndrome.2023; 32(4): 289. CrossRef - Sodium-glucose cotransporter 2 inhibitors for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: A nationwide propensity-score matched cohort study
Jinyoung Kim, Kyungdo Han, Bongsung Kim, Ki-Hyun Baek, Ki-Ho Song, Mee Kyoung Kim, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2022; 194: 110187. CrossRef
- Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study

- Diabetes, Obesity and Metabolism
- Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2022;37(1):74-83. Published online February 9, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1293
- 4,937 View
- 235 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
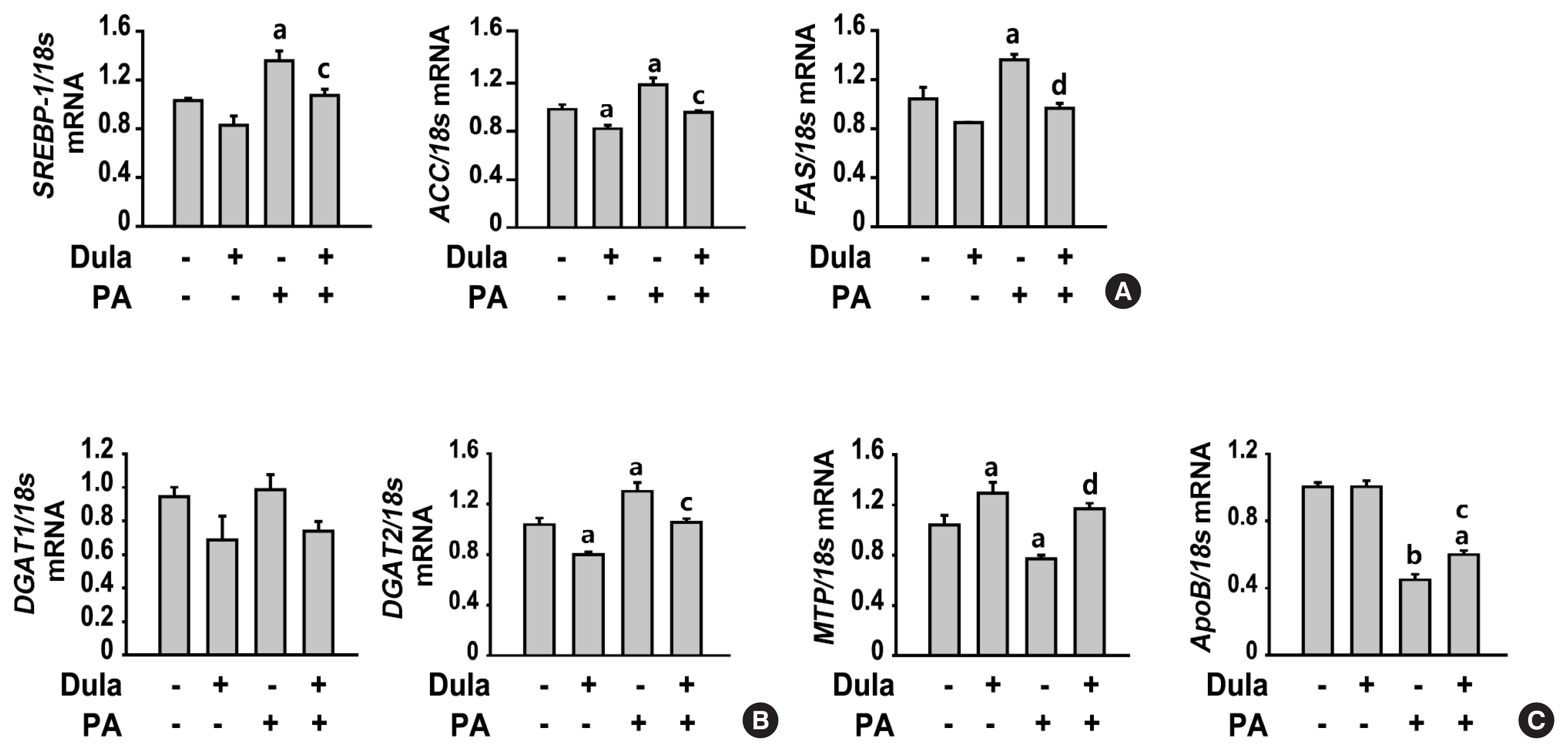
ePub - Background
Dulaglutide, a long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA), has been shown to reduce body weight and liver fat content in patients with type 2 diabetes. Family with sequence similarity 3 member A (FAM3A) plays a vital role in regulating glucose and lipid metabolism. The aim of this study was to determine the mechanisms by which dulaglutide protects against hepatic steatosis in HepG2 cells treated with palmitic acid (PA).
Methods
HepG2 cells were pretreated with 400 μM PA for 24 hours, followed by treatment with or without 100 nM dulaglutide for 24 hours. Hepatic lipid accumulation was determined using Oil red O staining and triglyceride (TG) assay, and the expression of lipid metabolism-associated factor was analyzed using quantitative real time polymerase chain reaction and Western blotting.
Results
Dulaglutide significantly decreased hepatic lipid accumulation and reduced the expression of genes associated with lipid droplet binding proteins, de novo lipogenesis, and TG synthesis in PA-treated HepG2 cells. Dulaglutide also increased the expression of proteins associated with lipolysis and fatty acid oxidation and FAM3A in PA-treated cells. However, exendin-(9-39), a GLP-1R antagonist, reversed the expression of FAM3A, and fatty acid oxidation-associated factors increased due to dulaglutide. In addition, inhibition of FAM3A by siRNA attenuated the reducing effect of dulaglutide on TG content and its increasing effect on regulation of fatty acid oxidation.
Conclusion
These results suggest that dulaglutide could be used therapeutically for improving nonalcoholic fatty liver disease, and its effect could be mediated in part via upregulation of FAM3A expression through a GLP-1R-dependent pathway. -
Citations
Citations to this article as recorded by- GLP-1/GLP-1RAs: New Options for the Drug Treatment of NAFLD
Haoran Jiang, Linquan Zang
Current Pharmaceutical Design.2024; 30(2): 100. CrossRef - GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives
Riccardo Nevola, Raffaella Epifani, Simona Imbriani, Giovanni Tortorella, Concetta Aprea, Raffaele Galiero, Luca Rinaldi, Raffaele Marfella, Ferdinando Carlo Sasso
International Journal of Molecular Sciences.2023; 24(2): 1703. CrossRef - FAM3A mediates the phenotypic switch of human aortic smooth muscle cells stimulated with oxidised low-density lipoprotein by influencing the PI3K-AKT pathway
Lei Yang, Baoshun Du, Shitao Zhang, Maode Wang
In Vitro Cellular & Developmental Biology - Animal.2023; 59(6): 431. CrossRef - ATP Secretion and Metabolism in Regulating Pancreatic Beta Cell Functions and Hepatic Glycolipid Metabolism
Jing Li, Han Yan, Rui Xiang, Weili Yang, Jingjing Ye, Ruili Yin, Jichun Yang, Yujing Chi
Frontiers in Physiology.2022;[Epub] CrossRef - Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH)
Xiaohan Xu, Kyle L. Poulsen, Lijuan Wu, Shan Liu, Tatsunori Miyata, Qiaoling Song, Qingda Wei, Chenyang Zhao, Chunhua Lin, Jinbo Yang
Signal Transduction and Targeted Therapy.2022;[Epub] CrossRef
- GLP-1/GLP-1RAs: New Options for the Drug Treatment of NAFLD

- Diabetes, Obesity and Metabolism
- The Effects of PPAR Agonists on Atherosclerosis and Nonalcoholic Fatty Liver Disease in ApoE−/−FXR−/− Mice
- Yenna Lee, Bo-Rahm Kim, Geun-Hyung Kang, Gwan Jae Lee, Young Joo Park, Haeryoung Kim, Hak Chul Jang, Sung Hee Choi
- Endocrinol Metab. 2021;36(6):1243-1253. Published online December 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1100

- 5,662 View
- 159 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Farnesoid X receptor (FXR), a bile acid–activated nuclear receptor, is a potent regulator of glucose and lipid metabolism as well as of bile acid metabolism. Previous studies have demonstrated that FXR deficiency is associated with metabolic derangements, including atherosclerosis and nonalcoholic fatty liver disease (NAFLD), but its mechanism remains unclear. In this study, we investigated the role of FXR in atherosclerosis and NAFLD and the effect of peroxisome proliferator-activated receptor (PPAR) agonists in mouse models with FXR deficiency.
Methods
En face lipid accumulation analysis, liver histology, serum levels of glucose and lipids, and mRNA expression of genes related to lipid metabolism were compared between apolipoprotein E (ApoE)−/− and ApoE−/−FXR−/− mice. The effects of PPARα and PPARγ agonists were also compared in both groups of mice.
Results
Compared with ApoE−/− mice, ApoE−/−FXR−/− mice showed more severe atherosclerosis, hepatic steatosis, and higher levels of serum cholesterol, low-density lipoprotein cholesterol, and triglycerides, accompanied by increased mRNA expression of FAS, ApoC2, TNFα, IL-6 (liver), ATGL, TGH, HSL, and MGL (adipocytes), and decreased mRNA expressions of CPT2 (liver) and Tfam (skeletal muscle). Treatment with a PPARα agonist, but not with a PPARγ agonist, partly reversed atherosclerosis and hepatic steatosis, and decreased plasma triglyceride levels in the ApoE−/−FXR−/− mice, in association with increased mRNA expression of CD36 and FATP and decreased expression of ApoC2 and ApoC3 (liver).
Conclusion
Loss of FXR is associated with aggravation of atherosclerosis and hepatic steatosis in ApoE-deficient mice, which could be reversed by a PPARα agonist through induction of fatty acid uptake, β-oxidation, and triglyceride hydrolysis. -
Citations
Citations to this article as recorded by- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model
Shu-Yan Gao, Jing-Cheng Zhao, Qing Xia, Chen Sun, Maimaiti Aili, Ainiwaer Talifu, Shi-Xia Huo, Yun Zhang, Zhi-Jian Li
Frontiers in Pharmacology.2024;[Epub] CrossRef - Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics
Yuxiao Jiang, Lili Wu, Xiaopeng Zhu, Hua Bian, Xin Gao, Mingfeng Xia
Lipids in Health and Disease.2024;[Epub] CrossRef - Mitochondrial carnitine palmitoyltransferase-II dysfunction: A possible novel mechanism for nonalcoholic fatty liver disease in hepatocarcinogenesis
Min Yao, Ping Zhou, Yan-Yan Qin, Li Wang, Deng-Fu Yao
World Journal of Gastroenterology.2023; 29(12): 1765. CrossRef - Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases
Tess Yntema, Debby P. Y. Koonen, Folkert Kuipers
Nutrients.2023; 15(8): 1850. CrossRef - The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease
Alexandra C. Finney, Sandeep Das, Dhananjay Kumar, M. Peyton McKinney, Bishuang Cai, Arif Yurdagul, Oren Rom
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Targeting PPARs for therapy of atherosclerosis: A review
Miao Miao, Xue Wang, Tian Liu, Yan-Jie Li, Wen-Qian Yu, Tong-Mei Yang, Shou-Dong Guo
International Journal of Biological Macromolecules.2023; 242: 125008. CrossRef - Cabernet sauvignon dry red wine ameliorates atherosclerosis in mice by regulating inflammation and endothelial function, activating AMPK phosphorylation, and modulating gut microbiota
Xinlong Cheng, Xue Han, Liangfu Zhou, Yasai Sun, Qian Zhou, Xuan Lin, Zhe Gao, Jie Wang, Wen Zhao
Food Research International.2023; 169: 112942. CrossRef - Impacts of dietary lipids derived from animal or vegetable sources on healthy rats
Mostafa M Dalal, Gamal M Edrees, Hanaa A Hassan, Mamdouh Abdel-Mogib, Mai Alaa El-Dein
Egyptian Journal of Basic and Applied Sciences.2023; 10(1): 618. CrossRef - Whey protein hydrolysate alleviated atherosclerosis and hepatic steatosis by regulating lipid metabolism in apoE-/- mice fed a Western diet
Kai Wang, Zixin Fu, Xiaoyi Li, Hui Hong, Xin Zhan, Xiaohong Guo, Yongkang Luo, Yuqing Tan
Food Research International.2022; 157: 111419. CrossRef - Melatonin alleviates PM2.5‐induced glucose metabolism disorder and lipidome alteration by regulating endoplasmic reticulum stress
Zhou Du, Junjie Hu, Lisen Lin, Qingqing Liang, Mengqi Sun, Zhiwei Sun, Junchao Duan
Journal of Pineal Research.2022;[Epub] CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef - The role of the gut microbiota in health and cardiovascular diseases
Lu Wang, Shiqi Wang, Qing Zhang, Chengqi He, Chenying Fu, Quan Wei
Molecular Biomedicine.2022;[Epub] CrossRef
- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model

- Diabetes, Obesity and Metabolism
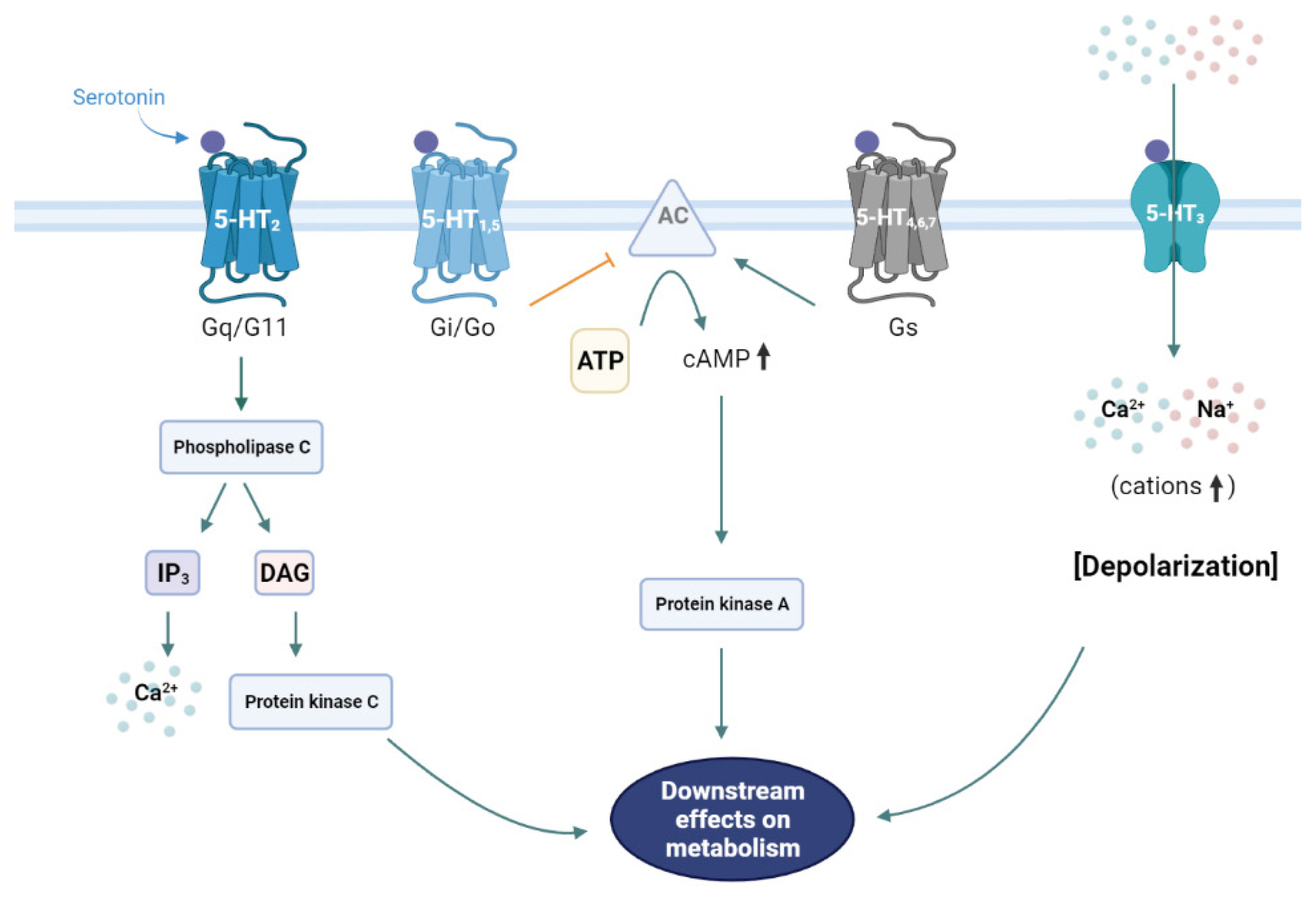
- Serotonergic Regulation of Hepatic Energy Metabolism
- Jiwon Park, Wooju Jeong, Chahyeon Yun, Hail Kim, Chang-Myung Oh
- Endocrinol Metab. 2021;36(6):1151-1160. Published online December 16, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1331
- 6,021 View
- 220 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The liver is a vital organ that regulates systemic energy metabolism and many physiological functions. Nonalcoholic fatty liver disease (NAFLD) is the commonest cause of chronic liver disease and end-stage liver failure. NAFLD is primarily caused by metabolic disruption of lipid and glucose homeostasis. Serotonin (5-hydroxytryptamine [5-HT]) is a biogenic amine with several functions in both the central and peripheral systems. 5-HT functions as a neurotransmitter in the brain and a hormone in peripheral tissues to regulate systemic energy homeostasis. Several recent studies have proposed various roles of 5-HT in hepatic metabolism and inflammation using tissue-specific knockout mice and 5-HT-receptor agonists/antagonists. This review compiles the most recent research on the relationship between 5-HT and hepatic metabolism, and the role of 5-HT signaling as a potential therapeutic target in NAFLD.
-
Citations
Citations to this article as recorded by- Maternal nutrient metabolism in the liver during pregnancy
Hongxu Fang, Qingyang Li, Haichao Wang, Ying Ren, Leying Zhang, Ling Yang
Frontiers in Endocrinology.2024;[Epub] CrossRef - Neural Stem Cell-based Regenerative Therapy: A New Approach to Diabetes
Treatment
Kajal Sharma, Nidhi Puranik, Dhananjay Yadav
Endocrine, Metabolic & Immune Disorders - Drug Targets.2024; 24(5): 531. CrossRef - Metabolic and Molecular Response to High-Fat Diet Differs between Rats with Constitutionally High and Low Serotonin Tone
Petra Baković, Maja Kesić, Darko Kolarić, Jasminka Štefulj, Lipa Čičin-Šain
International Journal of Molecular Sciences.2023; 24(3): 2169. CrossRef - Roles of gut microbes in metabolic-associated fatty liver disease
Chun-Yao Chen, Han-Chen Ho
Tzu Chi Medical Journal.2023; 35(4): 279. CrossRef - Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies
Kapil Kumar Goel, Somesh Thapliyal, Rajeev Kharb, Gaurav Joshi, Arvind Negi, Bhupinder Kumar
Pharmaceutics.2023; 15(9): 2208. CrossRef - Serotonin in the regulation of systemic energy metabolism
Joon Ho Moon, Chang‐Myung Oh, Hail Kim
Journal of Diabetes Investigation.2022; 13(10): 1639. CrossRef - Involvement of the liver-gut peripheral neural axis in nonalcoholic fatty liver disease pathologies via hepatic HTR2A
Takashi Owaki, Kenya Kamimura, Masayoshi Ko, Itsuo Nagayama, Takuro Nagoya, Osamu Shibata, Chiyumi Oda, Shinichi Morita, Atsushi Kimura, Takeki Sato, Toru Setsu, Akira Sakamaki, Hiroteru Kamimura, Takeshi Yokoo, Shuji Terai
Disease Models & Mechanisms.2022;[Epub] CrossRef - Non-alcoholic fatty liver disease (NAFLD) and mental illness: Mechanisms linking mood, metabolism and medicines
Anwesha Gangopadhyay, Radwa Ibrahim, Karli Theberge, Meghan May, Karen L. Houseknecht
Frontiers in Neuroscience.2022;[Epub] CrossRef
- Maternal nutrient metabolism in the liver during pregnancy

- Diabetes, Obesity and Metabolism
- The Leg Fat to Total Fat Ratio Is Associated with Lower Risks of Non-Alcoholic Fatty Liver Disease and Less Severe Hepatic Fibrosis: Results from Nationwide Surveys (KNHANES 2008–2011)
- Hyun Min Kim, Yong-ho Lee
- Endocrinol Metab. 2021;36(6):1232-1242. Published online November 23, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1087

- 3,850 View
- 131 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The prevalence of non-alcoholic fatty liver disease (NAFLD) has rapidly increased worldwide. The aim of this study was to investigate whether there is an independent relationship between regional fat distribution, especially leg fat mass, and the presence of NAFLD using nationally representative data in Korea.
Methods
This cross-sectional study analyzed data from 14,502 participants in the Korea National Health and Nutrition Examination Survey 2008 to 2011. Total fat mass, leg fat mass, and appendicular skeletal muscle mass were measured by dual-energy X-ray absorptiometry. Validated NAFLD prediction models and scoring systems for hepatic fibrosis were used.
Results
The leg fat to total fat (LF/TF) ratio showed a negative relationship with many factors, including body mass index, waist circumference, blood pressure, fasting blood glucose, and liver enzyme levels. When the LF/TF ratio and indices of hepatic steatosis were stratified by quartiles, the LF/TF ratio showed a negative correlation with the scoring systems that were used. The LF/TF ratio showed better accuracy in predicting NAFLD than total fat mass or leg fat mass alone. After adjusting for various traditional and lifestyle factors, a low LF/TF ratio remained a risk factor for NAFLD. Among NAFLD subjects, the LF/TF ratio showed a negative relationship with hepatic fibrosis.
Conclusion
A lower LF/TF ratio was markedly associated with a higher risk of hepatic steatosis and advanced hepatic fibrosis using various predictive models in a Korean population. Therefore, the LF/TF ratio could be a useful anthropometric parameter to predict NAFLD or advanced hepatic fibrosis. -
Citations
Citations to this article as recorded by- A greater ratio of thigh subcutaneous fat to abdominal fat is associated with protection against non-alcoholic fatty liver disease
Yebei Liang, Peizhu Chen, Siyu Chen, Dan Liu, Fusong Jiang, Zhijun Zhu, Keqing Dong, Li Wei, Xuhong Hou
JHEP Reports.2023; 5(7): 100730. CrossRef - Association between Alcohol Consumption and Metabolic Dysfunction-Associated Steatotic Liver Disease Based on Alcohol Flushing Response in Men: The Korea National Health and Nutrition Examination Survey 2019–2021
Dae Eon Kang, Si Nae Oh
Nutrients.2023; 15(18): 3901. CrossRef
- A greater ratio of thigh subcutaneous fat to abdominal fat is associated with protection against non-alcoholic fatty liver disease

- Diabetes, Obesity and Metabolism
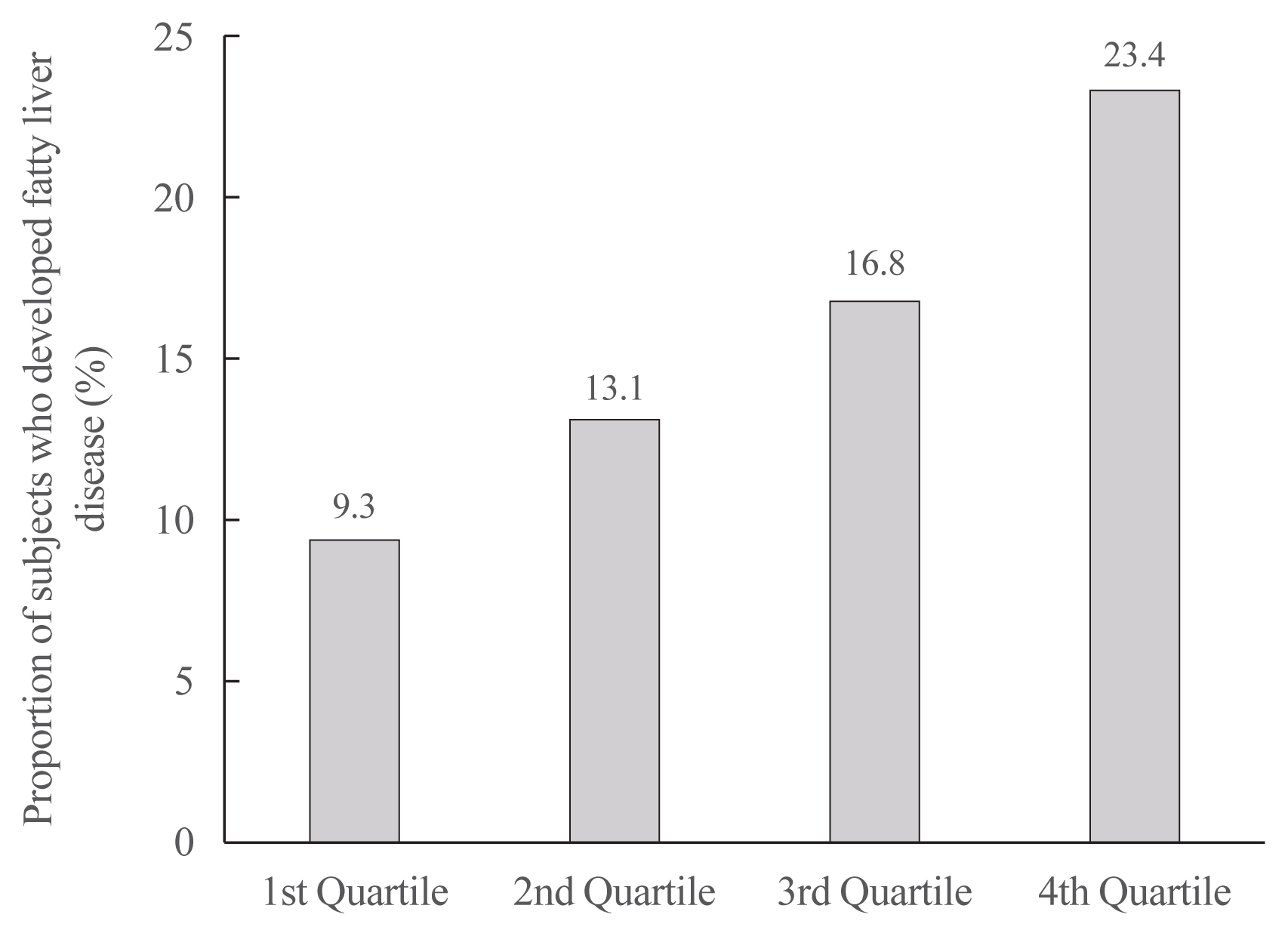
- Increased Risk of Nonalcoholic Fatty Liver Disease in Individuals with High Weight Variability
- Inha Jung, Dae-Jeong Koo, Mi Yeon Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2021;36(4):845-854. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1098
- 4,968 View
- 140 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Weight loss through lifestyle modification is recommended for patients with nonalcoholic fatty liver disease (NAFLD). Recent studies have suggested that repeated loss and gain of weight is associated with worse health outcomes. This study aimed to examine the association between weight variability and the risk of NAFLD in patients without diabetes.
Methods
We examined the health-checkup data of 30,708 participants who had undergone serial examinations between 2010 and 2014. Weight variability was assessed using coefficient of variation and the average successive variability of weight (ASVW), which was defined as the sum of absolute weight changes between successive years over the 5-year period divided by 4. The participants were classified according to the baseline body mass index and weight difference over 4 years.
Results
On dividing the participants into four groups according to ASVW quartile groups, those in the highest quartile showed a significantly increased risk of NAFLD compared to those in the lowest quartile (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.63 to 2.19). Among participants without obesity at baseline, individuals with high ASVW showed increased risk of NAFLD (OR, 1.80; 95% CI, 1.61 to 2.01). Participants with increased weight over 4 years and high ASVW demonstrated higher risk of NAFLD compared to those with stable weight and low ASVW (OR, 4.87; 95% CI, 4.29 to 5.53).
Conclusion
Regardless of participant baseline obesity status, high weight variability was associated with an increased risk of developing NAFLD. Our results suggest that further effort is required to minimize weight fluctuations after achieving a desirable body weight. -
Citations
Citations to this article as recorded by- Changes in Macronutrients during Dieting Lead to Weight Cycling and Metabolic Complications in Mouse Model
Anouk Charlot, Anthony Bringolf, Léa Debrut, Joris Mallard, Anne-Laure Charles, Emilie Crouchet, Delphine Duteil, Bernard Geny, Joffrey Zoll
Nutrients.2024; 16(5): 646. CrossRef - Body weight variability and the risk of liver‐related outcomes in type 2 diabetes and steatotic liver disease: a cohort study
Nathalie C. Leite, Claudia R. L. Cardoso, Cristiane A. Villela‐Nogueira, Gil F. Salles
Obesity.2024;[Epub] CrossRef - Weight variability, physical functioning and incident disability in older adults
Katie J. McMenamin, Tamara B. Harris, Joshua F. Baker
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(4): 1648. CrossRef - Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2022; 37(1): 74. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight
Mohammed Eslam, Hashem B. El-Serag, Sven Francque, Shiv K. Sarin, Lai Wei, Elisabetta Bugianesi, Jacob George
Nature Reviews Gastroenterology & Hepatology.2022; 19(10): 638. CrossRef - Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study
Ángel Arturo López-González, Bárbara Altisench Jané, Luis Masmiquel Comas, Sebastiana Arroyo Bote, Hilda María González San Miguel, José Ignacio Ramírez Manent
Nutrients.2022; 14(14): 2795. CrossRef - Higher Weight Variability Could Bring You a Fatty Liver
Yeoree Yang, Jae-Hyoung Cho
Endocrinology and Metabolism.2021; 36(4): 766. CrossRef - Autonomic Imbalance Increases the Risk for Non-alcoholic Fatty Liver Disease
Inha Jung, Da Young Lee, Mi Yeon Lee, Hyemi Kwon, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Won-Young Lee, Sung-Woo Park, Se Eun Park
Frontiers in Endocrinology.2021;[Epub] CrossRef
- Changes in Macronutrients during Dieting Lead to Weight Cycling and Metabolic Complications in Mouse Model

- Diabetes, Obesity and Metabolism
- Non-Laboratory-Based Simple Screening Model for Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Developed Using Multi-Center Cohorts
- Jiwon Kim, Minyoung Lee, Soo Yeon Kim, Ji-Hye Kim, Ji Sun Nam, Sung Wan Chun, Se Eun Park, Kwang Joon Kim, Yong-ho Lee, Joo Young Nam, Eun Seok Kang
- Endocrinol Metab. 2021;36(4):823-834. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1074
- 4,487 View
- 138 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of chronic liver disease worldwide. Type 2 diabetes mellitus (T2DM) is a risk factor that accelerates NAFLD progression, leading to fibrosis and cirrhosis. Thus, here we aimed to develop a simple model to predict the presence of NAFLD based on clinical parameters of patients with T2DM.
Methods
A total of 698 patients with T2DM who visited five medical centers were included. NAFLD was evaluated using transient elastography. Univariate logistic regression analyses were performed to identify potential contributors to NAFLD, followed by multivariable logistic regression analyses to create the final prediction model for NAFLD.
Results
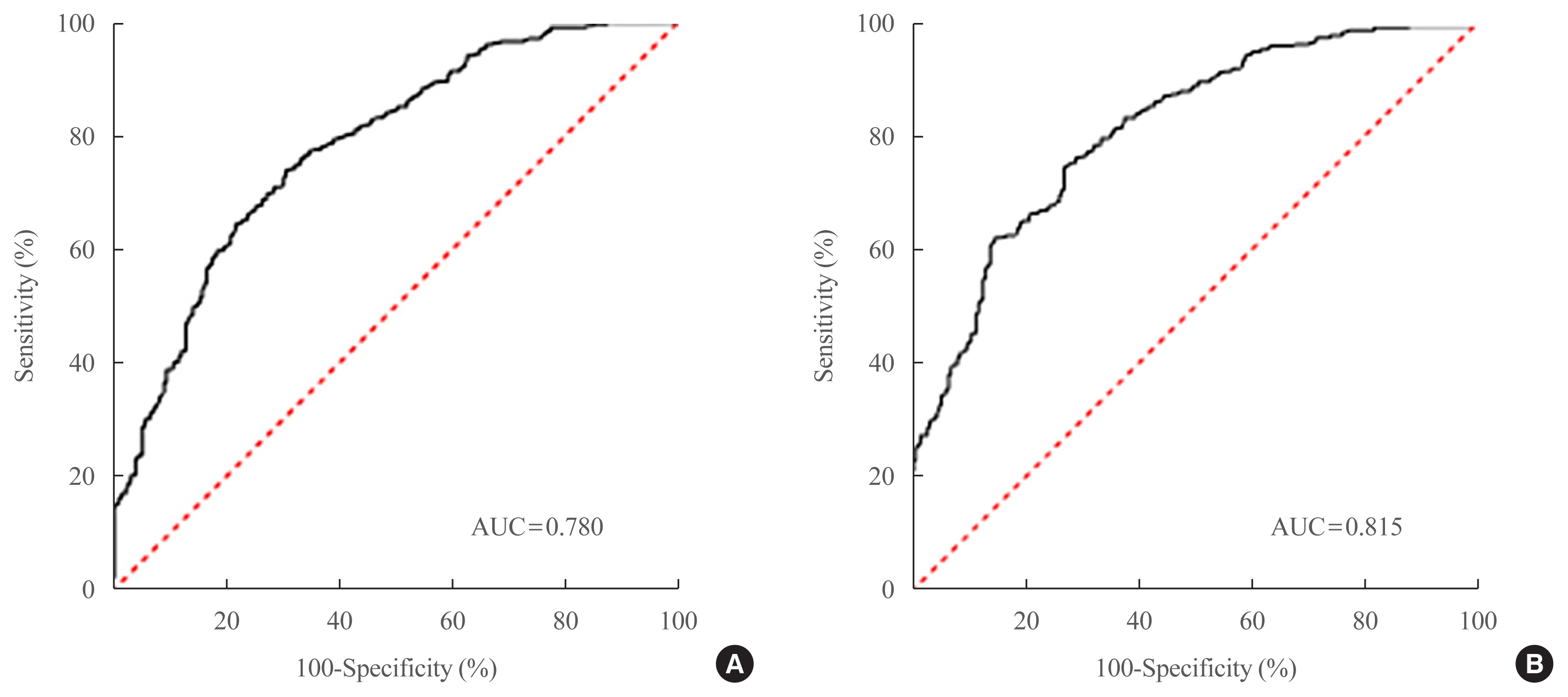
Two NAFLD prediction models were developed, with and without serum biomarker use. The non-laboratory model comprised six variables: age, sex, waist circumference, body mass index (BMI), dyslipidemia, and smoking status. For a cutoff value of ≥60, the prediction accuracy was 0.780 (95% confidence interval [CI], 0.743 to 0.817). The second comprehensive model showed an improved discrimination ability of up to 0.815 (95% CI, 0.782 to 0.847) and comprised seven variables: age, sex, waist circumference, BMI, glycated hemoglobin, triglyceride, and alanine aminotransferase to aspartate aminotransferase ratio. Our non-laboratory model showed non-inferiority in the prediction of NAFLD versus previously established models, including serum parameters.
Conclusion
The new models are simple and user-friendly screening methods that can identify individuals with T2DM who are at high-risk for NAFLD. Additional studies are warranted to validate these new models as useful predictive tools for NAFLD in clinical practice. -
Citations
Citations to this article as recorded by- Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus—The Chicken or the Egg Dilemma
Marcin Kosmalski, Agnieszka Śliwińska, Józef Drzewoski
Biomedicines.2023; 11(4): 1097. CrossRef
- Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus—The Chicken or the Egg Dilemma

- Diabetes, Obesity and Metabolism
- High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study
- Sung-Woo Kim, Jae-Han Jeon, Jun Sung Moon, Mi Kyung Kim
- Endocrinol Metab. 2021;36(4):800-809. Published online August 20, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1040
- 5,108 View
- 175 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Based on recent evidence on the importance of the presence of diabetes mellitus (DM) and fibrosis-4 (FIB-4) index in coronavirus disease 2019 (COVID-19) mortality, we analyzed whether these factors could additively predict such mortality.
Methods
This multicenter observational study included 1,019 adult inpatients admitted to university hospitals in Daegu. The demographic and laboratory findings, mortality, prevalence of severe disease, and duration of quarantine were compared between patients with and without DM and/or a high FIB-4 index. The mortality risk and corresponding hazard ratio (HR) were analyzed using the Kaplan-Meier method and Cox proportional hazard models.
Results
The patients with DM (n=217) exhibited significantly higher FIB-4 index and mortality compared to those without DM. Although DM (HR, 2.66; 95% confidence interval [CI], 1.63 to 4.33) and a high FIB-4 index (HR, 4.20; 95% CI, 2.21 to 7.99) were separately identified as risk factors for COVID-19 mortality, the patients with both DM and high FIB-4 index had a significantly higher mortality (HR, 9.54; 95% CI, 4.11 to 22.15). Higher FIB-4 indices were associated with higher mortality regardless of DM. A high FIB-4 index with DM was more significantly associated with a severe clinical course with mortality (odds ratio, 11.24; 95% CI, 5.90 to 21.41) than a low FIB-4 index without DM, followed by a high FIB-4 index alone and DM alone. The duration of quarantine and hospital stay also tended to be longer in those with both DM and high FIB-4 index.
Conclusion
Both DM and high FIB-4 index are independent and additive risk factors for COVID-19 mortality. -
Citations
Citations to this article as recorded by- COVID-19 and hepatic injury: Diversity and risk assessment
Fares E M Ali, Mostafa K Abd El-Aziz, Mahmoud M Ali, Osama M Ghogar, Adel G Bakr
World Journal of Gastroenterology.2023; 29(3): 425. CrossRef - Differential Effects of COVID-19 Hospitalization on the Trajectory of Liver Disease Progression
Dilara Hatipoğlu, Connor Mulligan, Jeffrey Wang, Juan Peticco, Reid Grinspoon, Sanjay Gadi, Camilla Mills, Jay Luther, Raymond T. Chung
Gastro Hep Advances.2023; 2(4): 480. CrossRef - Association of non-alcoholic fatty liver and metabolic-associated fatty liver with COVID-19 outcomes: A systematic review and meta-analysis
Gowthami Sai Kogilathota Jagirdhar, Rakhtan K Qasba, Harsha Pattnaik, Kaanthi Rama, Akshat Banga, Shiva Teja Reddy, Anna Carolina Flumignan Bucharles, Rahul Kashyap, Praveen Reddy Elmati, Vikas Bansal, Yatinder Bains, Theodore DaCosta, Salim Surani
World Journal of Gastroenterology.2023; 29(21): 3362. CrossRef - COVID-19 and Fatty Liver Disorders
Maria Guarino, Valentina Cossiga, Francesco Cutolo, Maria Attanasio, Raffaele Lieto, Filomena Morisco
Journal of Clinical Medicine.2023; 12(13): 4316. CrossRef - Prevalence and Prognostic Significance of Liver Fibrosis in Patients With Aneurysmal Subarachnoid Hemorrhage
Tiangui Li, Peng Wang, Xiao Gong, Weelic Chong, Yang Hai, Chao You, Juan Kang, Fang Fang, Yu Zhang
Frontiers in Neurology.2022;[Epub] CrossRef
- COVID-19 and hepatic injury: Diversity and risk assessment

- Clinical Study
- Development of a Non-Invasive Liver Fibrosis Score Based on Transient Elastography for Risk Stratification in Patients with Type 2 Diabetes
- Chi-Ho Lee, Wai-Kay Seto, Kelly Ieong, David T.W. Lui, Carol H.Y. Fong, Helen Y. Wan, Wing-Sun Chow, Yu-Cho Woo, Man-Fung Yuen, Karen S.L. Lam
- Endocrinol Metab. 2021;36(1):134-145. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.887
- 4,538 View
- 132 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
In non-alcoholic fatty liver disease (NAFLD), transient elastography (TE) is an accurate non-invasive method to identify patients at risk of advanced fibrosis (AF). We developed a diabetes-specific, non-invasive liver fibrosis score based on TE to facilitate AF risk stratification, especially for use in diabetes clinics where TE is not readily available.
Methods
Seven hundred sixty-six adults with type 2 diabetes and NAFLD were recruited and randomly divided into a training set (n=534) for the development of diabetes fibrosis score (DFS), and a testing set (n=232) for internal validation. DFS identified patients with AF on TE, defined as liver stiffness (LS) ≥9.6 kPa, based on a clinical model comprising significant determinants of LS with the lowest Akaike information criteria. The performance of DFS was compared with conventional liver fibrosis scores (NFS, FIB-4, and APRI), using area under the receiver operating characteristic curve (AUROC), sensitivity, specificity, positive and negative predictive values (NPV).
Results
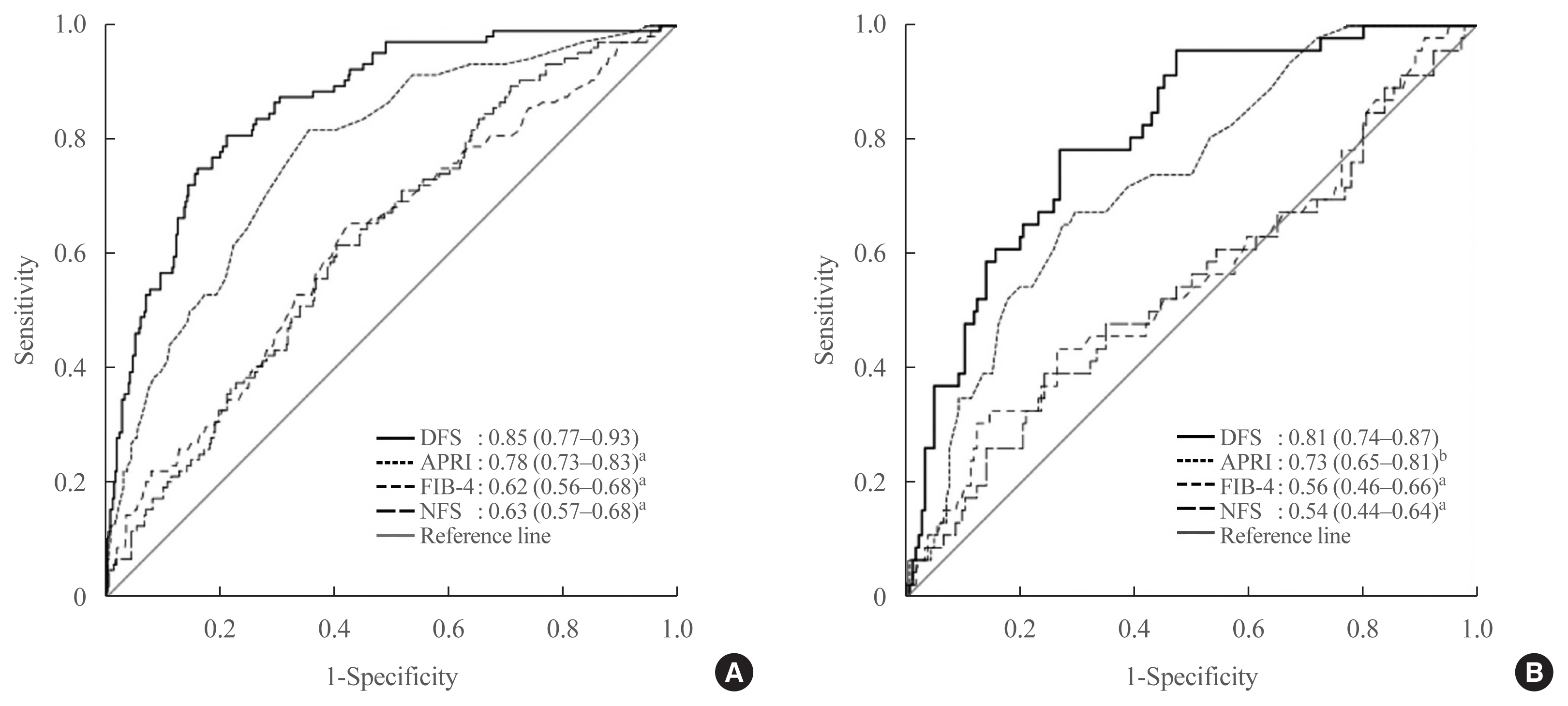
DFS comprised body mass index, platelet, aspartate aminotransferase, high-density lipoprotein cholesterol, and albuminuria, five routine measurements in standard diabetes care. Derived low and high DFS cut-offs were 0.1 and 0.3, with 90% sensitivity and 90% specificity, respectively. Both cut-offs provided better NPVs of >90% than conventional fibrosis scores. The AUROC of DFS for AF on TE was also higher (P<0.01) than the conventional fibrosis scores, being 0.85 and 0.81 in the training and testing sets, respectively.
Conclusion
Compared to conventional fibrosis scores, DFS, with a high NPV, more accurately identified diabetes patients at-risk of AF, who need further evaluation by hepatologists. -
Citations
Citations to this article as recorded by- Implementation of a liver health check in people with type 2 diabetes
Kushala W M Abeysekera, Luca Valenti, Zobair Younossi, John F Dillon, Alina M Allen, Mazen Noureddin, Mary E Rinella, Frank Tacke, Sven Francque, Pere Ginès, Maja Thiele, Philip N Newsome, Indra Neil Guha, Mohammed Eslam, Jörn M Schattenberg, Saleh A Alqa
The Lancet Gastroenterology & Hepatology.2024; 9(1): 83. CrossRef - Sequential algorithm to stratify liver fibrosis risk in overweight/obese metabolic dysfunction-associated fatty liver disease
Chi-Ho Lee, David Tak-Wai Lui, Raymond Hang-Wun Li, Michele Mae-Ann Yuen, Carol Ho-Yi Fong, Ambrose Pak-Wah Leung, Justin Chiu-Man Chu, Loey Lung-Yi Mak, Tai-Hing Lam, Jean Woo, Yu-Cho Woo, Aimin Xu, Hung-Fat Tse, Kathryn Choon-Beng Tan, Bernard Man-Yung
Frontiers in Endocrinology.2023;[Epub] CrossRef - Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review
Luca Rinaldi, Chiara Giorgione, Andrea Mormone, Francesca Esposito, Michele Rinaldi, Massimiliano Berretta, Raffaele Marfella, Ciro Romano
Viruses.2023; 15(8): 1730. CrossRef - Metabolic dysfunction-associated fatty liver disease — How relevant is this to primary care physicians and diabetologists?
Chi-Ho Lee
Primary Care Diabetes.2022; 16(2): 245. CrossRef - Non‐alcoholic fatty liver disease and type 2 diabetes: An update
Chi‐H Lee, David TW Lui, Karen SL Lam
Journal of Diabetes Investigation.2022; 13(6): 930. CrossRef - Ultrasound-Based Hepatic Elastography in Non-Alcoholic Fatty Liver Disease: Focus on Patients with Type 2 Diabetes
Georgiana-Diana Cazac, Cristina-Mihaela Lăcătușu, Cătălina Mihai, Elena-Daniela Grigorescu, Alina Onofriescu, Bogdan-Mircea Mihai
Biomedicines.2022; 10(10): 2375. CrossRef
- Implementation of a liver health check in people with type 2 diabetes

- Obesity and Metabolism
- Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease
- Dae Ho Lee
- Endocrinol Metab. 2020;35(2):243-259. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.243
- 11,133 View
- 299 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver diseases and can progress to advanced fibrosis and end-stage liver disease. Thus, intensive research has been performed to develop noninvasive methods for the diagnosis of nonalcoholic steatohepatitis (NASH) and fibrosis. Currently, no single noninvasive tool covers all of the stages of pathologies and conditions of NAFLD, and the cost and feasibility of known techniques are also important issues. Blood biomarkers for NAFLD may be useful to select subjects who need ultrasonography (US) screening for NAFLD, and noninvasive tools for assessing fibrosis may be helpful to exclude the probability of significant fibrosis and to predict advanced fibrosis, thus guiding the decision of whether to perform liver biopsy in patients with NAFLD. Among various methods, magnetic resonance-based methods have been shown to perform better than other methods in assessing steatosis as well as in detecting hepatic fibrosis. Many genetic markers are associated with the development and progression of NAFLD. Further well-designed studies are needed to determine which biomarker panels, imaging studies, genetic marker panels, or combinations thereof perform well for diagnosing NAFLD, differentiating NASH and fibrosis, and following-up NAFLD, respectively.
-
Citations
Citations to this article as recorded by- Recent Progresses on Pathophysiology, Diagnosis, Therapeutic Modalities,
and Management of Non-alcoholic Fatty Liver Disorder
Mahdi Barazesh, Sajad Jalili, Morteza Akhzari, Fouzieyeh Faraji, Ebrahim Khorramdin
Current Drug Therapy.2024; 19(1): 20. CrossRef - Intact ketogenesis predicted reduced risk of moderate-severe metabolic-associated fatty liver disease assessed by liver transient elastography in newly diagnosed type 2 diabetes
Sejeong Lee, Jaehyun Bae, Seung Up Kim, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2024;[Epub] CrossRef - Association between nonalcoholic fatty liver disease and left ventricular diastolic dysfunction: A 7-year retrospective cohort study of 3,380 adults using serial echocardiography
Gyuri Kim, Tae Yang Yu, Jae Hwan Jee, Ji Cheol Bae, Mira Kang, Jae Hyeon Kim
Diabetes & Metabolism.2024; 50(3): 101534. CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
Monica A Tincopa, Rohit Loomba
The Lancet Gastroenterology & Hepatology.2023; 8(7): 660. CrossRef - Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning
Adelaida Solomon, Călin Remus Cipăian, Mihai Octavian Negrea, Adrian Boicean, Romeo Mihaila, Corina Beca, Mirela Livia Popa, Sebastian Mihai Grama, Minodora Teodoru, Bogdan Neamtu
Journal of Clinical Medicine.2023; 12(17): 5657. CrossRef - Greater Severity of Steatosis Is Associated with a Higher Risk of Incident Diabetes: A Retrospective Longitudinal Study
Ji Min Han, Jung Hwan Cho, Hye In Kim, Sunghwan Suh, Yu-Ji Lee, Jung Won Lee, Kwang Min Kim, Ji Cheol Bae
Endocrinology and Metabolism.2023; 38(4): 418. CrossRef - Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease
Georgiana-Emmanuela Gîlcă-Blanariu, Daniela Simona Budur, Dana Elena Mitrică, Elena Gologan, Oana Timofte, Gheorghe Gh Bălan, Vasile Andrei Olteanu, Gabriela Ștefănescu
Metabolites.2023; 13(11): 1115. CrossRef - Plasma Aldo-Keto Reductase Family 1 Member B10 as a Biomarker Performs Well in the Diagnosis of Nonalcoholic Steatohepatitis and Fibrosis
Aron Park, Seung Joon Choi, Sungjin Park, Seong Min Kim, Hye Eun Lee, Minjae Joo, Kyoung Kon Kim, Doojin Kim, Dong Hae Chung, Jae Been Im, Jaehun Jung, Seung Kak Shin, Byung-Chul Oh, Cheolsoo Choi, Seungyoon Nam, Dae Ho Lee
International Journal of Molecular Sciences.2022; 23(9): 5035. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - The Impact of Insulin Resistance on Hepatic Fibrosis among United States Adults with Non-Alcoholic Fatty Liver Disease: NHANES 2017 to 2018
Ji Cheol Bae, Lauren A. Beste, Kristina M. Utzschneider
Endocrinology and Metabolism.2022; 37(3): 455. CrossRef - Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis
Moongi Ji, Yunju Jo, Seung Joon Choi, Seong Min Kim, Kyoung Kon Kim, Byung-Chul Oh, Dongryeol Ryu, Man-Jeong Paik, Dae Ho Lee
Biomedicines.2022; 10(7): 1669. CrossRef - Evaluation of Liver Changes in Type-2 Diabetes Mellitus Patients using Computed Tomography
Nayyar Ashfaq, Akash John, Abid Ali, Amina Sharif Bhatti, Hateem Qaiser
DIET FACTOR (Journal of Nutritional & Food Sciences).2022; : 14. CrossRef - Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers
Mauro Viganò, Nicola Pugliese, Federica Cerini, Federica Turati, Vincenzo Cimino, Sofia Ridolfo, Simone Rocchetto, Francesca Foglio, Maria Terrin, Carlo La Vecchia, Maria Grazia Rumi, Alessio Aghemo
International Journal of Molecular Sciences.2022; 23(20): 12489. CrossRef - MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy
Ying-Xin Xian, Jian-Ping Weng, Fen Xu
Chinese Medical Journal.2021; 134(1): 8. CrossRef - Prognostic accuracy of FIB‐4, NAFLD fibrosis score and APRI for NAFLD‐related events: A systematic review
Jenny Lee, Yasaman Vali, Jerome Boursier, Rene Spijker, Quentin M. Anstee, Patrick M. Bossuyt, Mohammad H. Zafarmand
Liver International.2021; 41(2): 261. CrossRef - Serum syndecan‐4 is associated with nonalcoholic fatty liver disease
Shu Jing Xia, Li Zhong Tang, Wen Hua Li, Zhao Shan Xu, Li Li Zhang, Feng Gan Cheng, Hong Xia Chen, Zi Hua Wang, Yu Cheng Luo, An Na Dai, Jian Gao Fan
Journal of Digestive Diseases.2021; 22(9): 536. CrossRef - Non-Laboratory-Based Simple Screening Model for Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Developed Using Multi-Center Cohorts
Jiwon Kim, Minyoung Lee, Soo Yeon Kim, Ji-Hye Kim, Ji Sun Nam, Sung Wan Chun, Se Eun Park, Kwang Joon Kim, Yong-ho Lee, Joo Young Nam, Eun Seok Kang
Endocrinology and Metabolism.2021; 36(4): 823. CrossRef - Pemafibrate Ameliorates Liver Dysfunction and Fatty Liver in Patients with Non-Alcoholic Fatty Liver Disease with Hypertriglyceridemia: A Retrospective Study with the Outcome after a Mid-Term Follow-Up
Suguru Ikeda, Takaaki Sugihara, Takuya Kihara, Yukako Matsuki, Takakazu Nagahara, Tomoaki Takata, Sonoko Kitao, Tsuyoshi Okura, Kazuhiro Yamamoto, Hajime Isomoto
Diagnostics.2021; 11(12): 2316. CrossRef - Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects
Hwi Seung Kim, Yun Kyung Cho, Eun Hee Kim, Min Jung Lee, Chang Hee Jung, Joong-Yeol Park, Hong-Kyu Kim, Woo Je Lee
Journal of Clinical Medicine.2021; 11(1): 41. CrossRef - The Leg Fat to Total Fat Ratio Is Associated with Lower Risks of Non-Alcoholic Fatty Liver Disease and Less Severe Hepatic Fibrosis: Results from Nationwide Surveys (KNHANES 2008–2011)
Hyun Min Kim, Yong-ho Lee
Endocrinology and Metabolism.2021; 36(6): 1232. CrossRef
- Recent Progresses on Pathophysiology, Diagnosis, Therapeutic Modalities,
and Management of Non-alcoholic Fatty Liver Disorder

- Clinical Study
- Visceral-to-Subcutaneous Abdominal Fat Ratio Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis
- Chan-Hee Jung, Eun-Jung Rhee, Hyemi Kwon, Yoosoo Chang, Seungho Ryu, Won-Young Lee
- Endocrinol Metab. 2020;35(1):165-176. Published online March 19, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.1.165
- 6,562 View
- 137 Download
- 26 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background We evaluated the association of visceral-to-subcutaneous fat ratio (VSR) with nonalcoholic fatty liver disease (NAFLD) and advanced fibrosis degree based on noninvasive serum fibrosis markers in the general population with NAFLD.
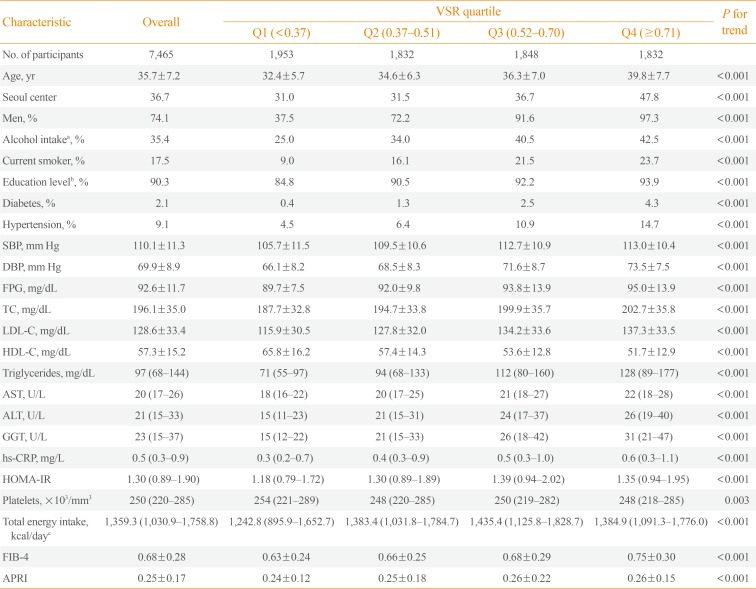
Methods This is a cross-sectional study, in 7,465 Korean adults who underwent health screening examinations. NAFLD was defined as fatty liver detected on ultrasonography, and visceral and subcutaneous abdominal fat was measured using computed tomography. We predicted fibrosis based on the fibrosis-4 (FIB-4) score and aspartate aminotransferase-to-platelet ratio index (APRI) and categorized the risk for advanced fibrosis as low, indeterminate, or high.
Results The multivariable-adjusted prevalence ratios for indeterminate to high risk of advanced fibrosis based on FIB-4, determined by comparing the second, third, and fourth quartiles with the first quartile of VSR, were 3.38 (95% confidence interval [CI], 0.64 to 17.97), 9.41 (95% CI, 1.97 to 45.01), and 19.34 (95% CI, 4.06 to 92.18), respectively. The multivariable-adjusted prevalence ratios for intermediate to high degree of fibrosis according to APRI also increased across VSR quartiles (5.04 [95% CI, 2.65 to 9.59], 7.51 [95% CI, 3.91 to 14.42], and 19.55 [95% CI, 9.97 to 38.34], respectively). High VSR was more strongly associated with the prevalence of NAFLD in nonobese subjects than in obese subjects, and the associations between VSR and intermediate to high probability of advanced fibrosis in NAFLD were stronger in obese subjects than in nonobese subjects.
Conclusion High VSR values predicted increased NAFLD risk and advanced fibrosis risk with NAFLD, and the predictive value of VSR for indeterminate to high risk of advanced fibrosis was higher in obese subjects than in nonobese subjects.
-
Citations
Citations to this article as recorded by- Opportunistic Extraction of Quantitative CT Biomarkers: Turning the Incidental Into Prognostic Information
Mohammad Nazri Md Shah, Raja Rizal Azman, Wai Yee Chan, Kwan Hoong Ng
Canadian Association of Radiologists Journal.2024; 75(1): 92. CrossRef - Positive Association Between the Chinese Visceral Adiposity Index and Nonalcoholic Fatty Liver Disease in Lean Adults
Shuxia Shen, Hangkai Huang, Jinghua Wang, Zexi Tang, Chao Shen, Chengfu Xu
Digestive Diseases and Sciences.2023; 68(2): 656. CrossRef - Association between Sarcopenic Obesity Status and Nonalcoholic Fatty Liver Disease and Fibrosis
Wolhwa Song, Sung Hwan Yoo, Jinsun Jang, Su Jung Baik, Byoung Kwon Lee, Hyun Woong Lee, Jong Suk Park
Gut and Liver.2023; 17(1): 130. CrossRef - Using hyperhomocysteinemia and body composition to predict the risk of non-alcoholic fatty liver disease in healthcare workers
Xiaoyan Hao, Honghai He, Liyuan Tao, Peng Wang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Visceral and subcutaneous fat, muscle mass, and liver volume as noninvasive predictors of the progress of non-alcoholic fatty liver disease
Omar M. Mahmoud, Gehad Abd Elaziz Mahmoud, Haisam Atta, Wael A. Abbas, Hanan M. Ahmed, Mohamed A. A. Abozaid
Egyptian Journal of Radiology and Nuclear Medicine.2023;[Epub] CrossRef - Relationship between metabolic associated fatty liver disease and body fat ratio, visceral fat area, and resting metabolic rate estimated by bioelectrical impedance analysis
Deng-Hua He, Yong-Zhan Zhang, Liang Xu, Jia-Jia Pei, Ying Zhang, Zhong-Fang Yan
World Chinese Journal of Digestology.2023; 31(2): 56. CrossRef - Poor glycaemic control and ectopic fat deposition mediates the increased risk of non-alcoholic steatohepatitis in high-risk populations with type 2 diabetes: Insights from Bayesian-network modelling
T. Waddell, A. Namburete, P. Duckworth, A. Fichera, A. Telford, H. Thomaides-Brears, D. J. Cuthbertson, M. Brady
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Subcutaneous Fat Obesity in a High Body Mass Index Donor Is Not a Contraindication to Living Donor Hepatectomy
Hirak Pahari, Amey Sonavane, Amruth Raj, Anup Kumar Agrawal, Ambreen Sawant, Deepak Kumar Gupta, Amit Gharat, Vikram Raut, Sorabh Kapoor
Case Reports in Hepatology.2023; 2023: 1. CrossRef - Comparison of cardiometabolic risk factors between obese and non-obese patients with nonalcoholic fatty liver disease
Zahra Yari, Danial Fotros, Azita Hekmatdoost
Scientific Reports.2023;[Epub] CrossRef - Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity
Hong-Kyu Kim, Sung-Jin Bae, Min Jung Lee, Eun Hee Kim, Hana Park, Hwi Seung Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee, Jaewon Choe
Clinical and Molecular Hepatology.2023; 29(4): 987. CrossRef - Visceral Adipose Tissue Inflammation and Radiographic Visceral-to-Subcutaneous Adipose Tissue Ratio in Patients with Cirrhosis
Nghiem B. Ha, Soo-Jin Cho, Yara Mohamad, Dorothea Kent, Grace Jun, Randi Wong, Vivek Swarnakar, Shezhang Lin, Jacquelyn J. Maher, Jennifer C. Lai
Digestive Diseases and Sciences.2022; 67(7): 3436. CrossRef - The Influence of Obesity and Metabolic Health on Vascular Health
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(1): 1. CrossRef - The effect of combined exercises on the plasma levels of retinol-binding protein 4 and its relationship with insulin resistance and hepatic fat content in postmenopausal women with nonalcoholic fatty liver disease
Masoumeh NOROUZPOUR, Sayyed M. MARANDI, Mohsen GHANBARZADEH, Abbasali ZARE MAIVAN
The Journal of Sports Medicine and Physical Fitness.2022;[Epub] CrossRef - The Perirenal Fat Thickness Was Associated with Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus
Yuxian Yang, Shuting Li, Yuechao Xu, Jing Ke, Dong Zhao
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 1505. CrossRef - Visceral adiposity is an independent risk factor for high intra-operative blood loss during living-donor liver transplantation; could preoperative rehabilitation and nutritional therapy mitigate that risk?
Mahmoud Macshut, Toshimi Kaido, Siyuan Yao, Yosuke Miyachi, Mohamed Sharshar, Sena Iwamura, Masaaki Hirata, Hisaya Shirai, Naoko Kamo, Shintaro Yagi, Shinji Uemoto
Clinical Nutrition.2021; 40(3): 956. CrossRef - A review of non‐alcoholic fatty liver disease in non‐obese and lean individuals
Mitra Ahadi, Kasra Molooghi, Negin Masoudifar, Ali Beheshti Namdar, Hassan Vossoughinia, Mohammadreza Farzanehfar
Journal of Gastroenterology and Hepatology.2021; 36(6): 1497. CrossRef - Quantification of abdominal fat from computed tomography using deep learning and its association with electronic health records in an academic biobank
Matthew T MacLean, Qasim Jehangir, Marijana Vujkovic, Yi-An Ko, Harold Litt, Arijitt Borthakur, Hersh Sagreiya, Mark Rosen, David A Mankoff, Mitchell D Schnall, Haochang Shou, Julio Chirinos, Scott M Damrauer, Drew A Torigian, Rotonya Carr, Daniel J Rader
Journal of the American Medical Informatics Association.2021; 28(6): 1178. CrossRef - Hepatic Steatosis in Patients With Single Ventricle and a Fontan Circulation
David A. Katz, Daniel Peck, Adam M. Lubert, Mathias Possner, Faizeen Zafar, Andrew T. Trout, Joseph J. Palermo, Nadeem Anwar, Jonathan R. Dillman, Adam W. Powell, Stavra A. Xanthakos, Alexander R. Opotowsky, Gruschen Veldtman, Tarek Alsaied
Journal of the American Heart Association.2021;[Epub] CrossRef - Superficial vs Deep Subcutaneous Adipose Tissue: Sex-Specific Associations With Hepatic Steatosis and Metabolic Traits
Tessa Brand, Inge Christina Lamberta van den Munckhof, Marinette van der Graaf, Kiki Schraa, Helena Maria Dekker, Leonardus Antonius Bernardus Joosten, Mihai Gheorghe Netea, Niels Peter Riksen, Jacqueline de Graaf, Joseph Henricus Wilhelmus Rutten
The Journal of Clinical Endocrinology & Metabolism.2021; 106(10): e3881. CrossRef - Baseline homeostasis model assessment of insulin resistance associated with fibrosis progression in patients with nonalcoholic fatty liver disease without diabetes: A cohort study
Dae-Jeong Koo, Mi Yeon Lee, Inha Jung, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee, Ming-Lung Yu
PLOS ONE.2021; 16(8): e0255535. CrossRef - Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non‐alcoholic fatty liver disease assessed by magnetic resonance imaging
Anne Flint, Grit Andersen, Paul Hockings, Lars Johansson, Anni Morsing, Mads Sundby Palle, Thomas Vogl, Rohit Loomba, Leona Plum‐Mörschel
Alimentary Pharmacology & Therapeutics.2021; 54(9): 1150. CrossRef - Effects of IL-33 on 3T3-L1 cells and obese mice models induced by a high-fat diet
Yue Kai, Jingtao Gao, Hu Liu, Yubing Wang, Chenrui Tian, Sheng Guo, Ling He, Min Li, Zhongwei Tian, Xiangfeng Song
International Immunopharmacology.2021; 101: 108209. CrossRef - Lipid Accumulation Product as an Index for Visceral Obesity and Cardiovascular Risk among a Sample of Obese Egyptian Women
Nayera E. Hassan, Sahar A. El-Masry, Gamila S. M. El-Saeed, Mohamed S. El Hussieny
Open Access Macedonian Journal of Medical Sciences.2021; 9(B): 1229. CrossRef - Combined Effects of Dyslipidemia and High Adiposity on the Estimated Glomerular Filtration Rate in a Middle-Aged Chinese Population
Xichang Wang, Haoyu Wang, Jiashu Li, Xiaotong Gao, Yutong Han, Weiping Teng, Zhongyan Shan, Yaxin Lai
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4513. CrossRef - Determination of “indeterminate score” measurements in lean nonalcoholic fatty liver disease patients from western Saudi Arabia
Yasir Mohammed Khayyat
World Journal of Hepatology.2021; 13(12): 2150. CrossRef - Utility of Liver Function Tests and Fatty Liver Index to Categorize Metabolic Phenotypes in a Mediterranean Population
Dariusz Narankiewicz, Josefina Ruiz-Nava, Veronica Buonaiuto, María Isabel Ruiz-Moreno, María Dolores López-Carmona, Luis Miguel Pérez-Belmonte, Ricardo Gómez-Huelgas, María Rosa Bernal-López
International Journal of Environmental Research and Public Health.2020; 17(10): 3518. CrossRef
- Opportunistic Extraction of Quantitative CT Biomarkers: Turning the Incidental Into Prognostic Information


 KES
KES

 First
First Prev
Prev



