Search
- Page Path
- HOME > Search
Original Articles
- Thyroid
- Prognostic Roles of Inflammatory Biomarkers in Radioiodine-Refractory Thyroid Cancer Treated with Lenvatinib
- Chae A Kim, Mijin Kim, Meihua Jin, Hee Kyung Kim, Min Ji Jeon, Dong Jun Lim, Bo Hyun Kim, Ho-Cheol Kang, Won Bae Kim, Dong Yeob Shin, Won Gu Kim
- Endocrinol Metab. 2024;39(2):334-343. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1854

- 971 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), serve as valuable prognostic indicators in various cancers. This multicenter, retrospective cohort study assessed the treatment outcomes of lenvatinib in 71 patients with radioactive iodine (RAI)-refractory thyroid cancer, considering the baseline inflammatory biomarkers.
Methods
This study retrospectively included patients from five tertiary hospitals in Korea whose complete blood counts were available before lenvatinib treatment. Progression-free survival (PFS) and overall survival (OS) were evaluated based on the median value of inflammatory biomarkers.
Results
No significant differences in baseline characteristics were observed among patients grouped according to the inflammatory biomarkers, except for older patients with a higher-than-median NLR (≥2) compared to their counterparts with a lower NLR (P= 0.01). Patients with a higher-than-median NLR had significantly shorter PFS (P=0.02) and OS (P=0.017) than those with a lower NLR. In multivariate analysis, a higher-than-median NLR was significantly associated with poor OS (hazard ratio, 3.0; 95% confidence interval, 1.24 to 7.29; P=0.015). However, neither the LMR nor the PLR was associated with PFS. A higher-than-median LMR (≥3.9) was significantly associated with prolonged OS compared to a lower LMR (P=0.036). In contrast, a higher-than-median PLR (≥142.1) was associated with shorter OS compared to a lower PLR (P=0.039).
Conclusion
Baseline inflammatory biomarkers can serve as predictive indicators of PFS and OS in patients with RAI-refractory thyroid cancer treated with lenvatinib.

- Thyroid
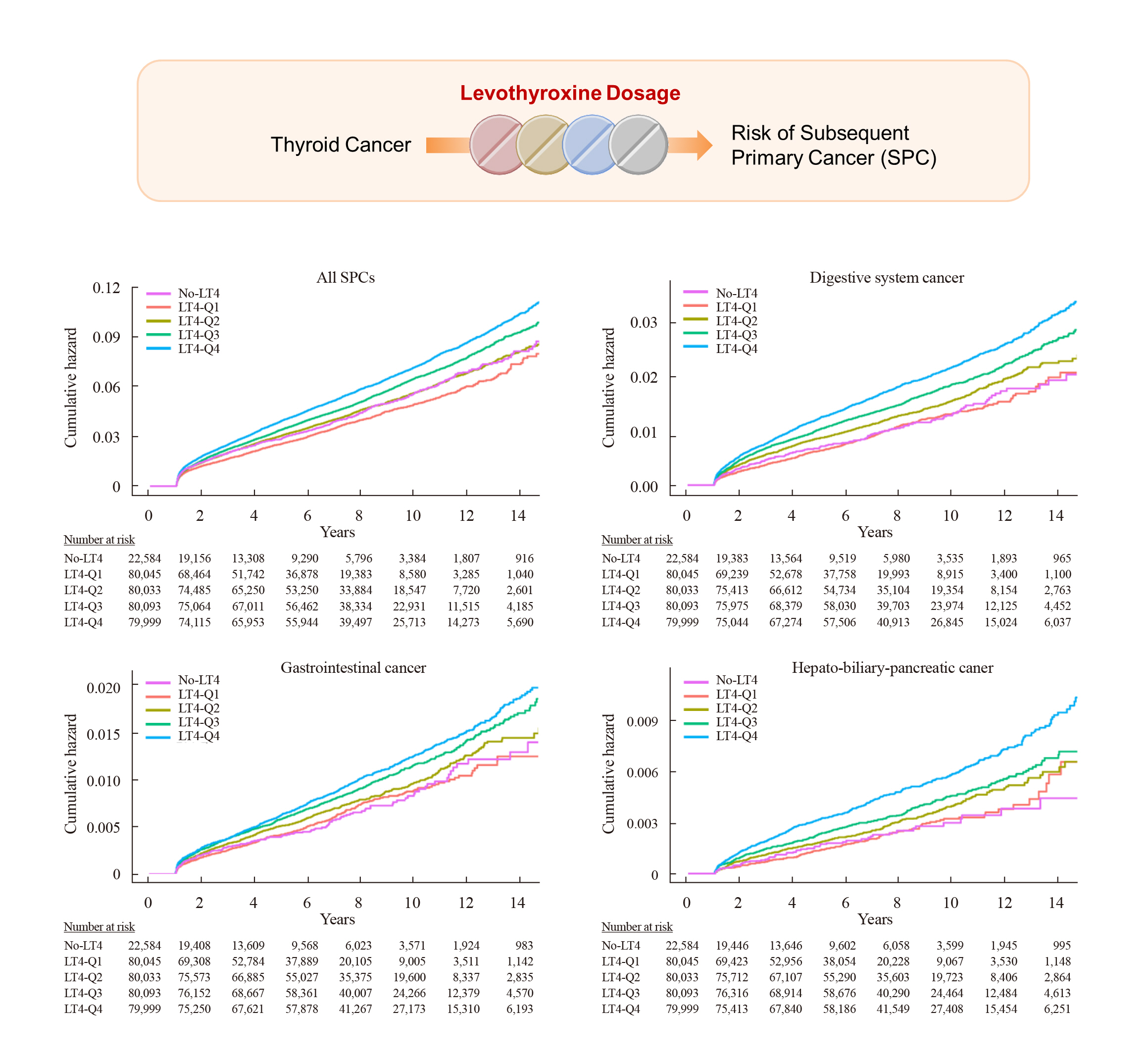
Big Data Articles (National Health Insurance Service Database) - Risk of Subsequent Primary Cancers in Thyroid Cancer Survivors according to the Dose of Levothyroxine: A Nationwide Cohort Study
- Min-Su Kim, Jang Won Lee, Min Kyung Hyun, Young Shin Song
- Endocrinol Metab. 2024;39(2):288-299. Published online March 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1815
- 1,386 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Current research has not investigated the effect of thyroid-stimulating hormone suppression therapy with levothyroxine on the risk for developing subsequent primary cancers (SPCs). This study aimed to investigate the association between levothyroxine dosage and the risk for SPCs in thyroid cancer patients.
Methods
We conducted a nationwide population-based retrospective cohort study form Korean National Health Insurance database. This cohort included 342,920 thyroid cancer patients between 2004 and 2018. Patients were divided into the non-levothyroxine and the levothyroxine groups, the latter consisting of four dosage subgroups according to quartiles. Cox proportional hazard models were performed to evaluate the risk for SPCs by adjusting for variables including cumulative doses of radioactive iodine (RAI) therapy.
Results
A total of 17,410 SPC cases were observed over a median 7.3 years of follow-up. The high-dose levothyroxine subgroups (Q3 and Q4) had a higher risk for SPC (adjusted hazard ratio [HR], 1.14 and 1.27; 95% confidence interval [CI], 1.05–1.24 and 1.17– 1.37; respectively) compared to the non-levothyroxine group. In particular, the adjusted HR of stomach (1.31), colorectal (1.60), liver and biliary tract (1.95), and pancreatic (2.48) cancers were increased in the Q4 subgroup. We consistently observed a positive association between high levothyroxine dosage per body weight and risk of SPCs, even after adjusting for various confounding variables. Moreover, similar results were identified in the stratified analyses according to thyroidectomy type and RAI therapy, as well as in a subgroup analysis of patients with good adherence.
Conclusion
High-dose levothyroxine use was associated with increased risk of SPCs among thyroid cancer patients regardless of RAI therapy.

Review Article
- Thyroid
- Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Min Joo Kim, Jae Hoon Moon, Eun Kyung Lee, Young Shin Song, Kyong Yeun Jung, Ji Ye Lee, Ji-hoon Kim, Kyungsik Kim, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2024;39(1):47-60. Published online February 15, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1937
- 2,425 View
- 180 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.
-
Citations
Citations to this article as recorded by- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef
- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules

Original Article
- Thyroid
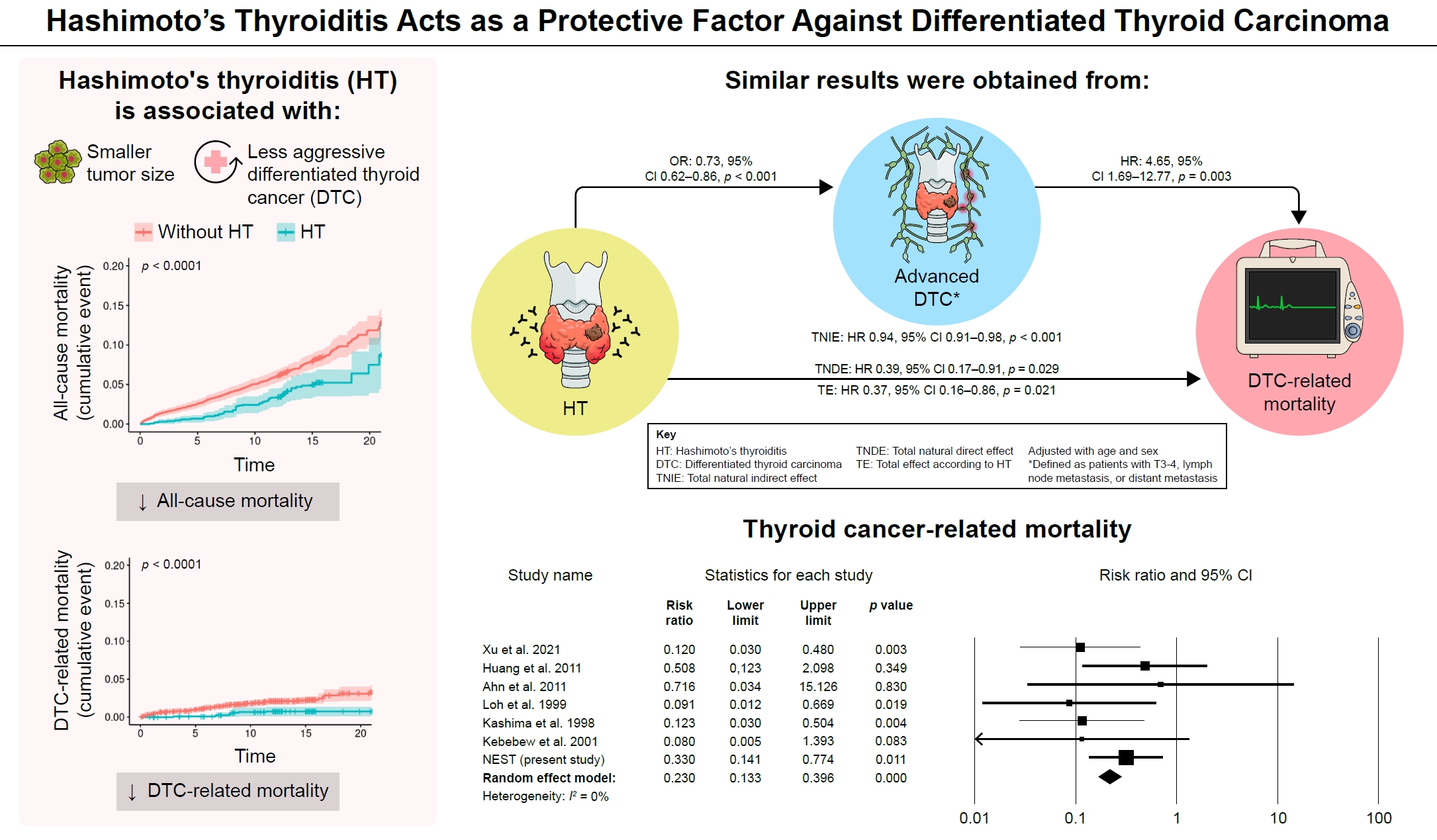
- Hashimoto Thyroiditis and Mortality in Patients with Differentiated Thyroid Cancer: The National Epidemiologic Survey of Thyroid Cancer in Korea and Meta-Analysis
- Injung Yang, Jae Myung Yu, Hye Soo Chung, Yoon Jung Kim, Yong Kyun Roh, Min Kyu Choi, Sung-ho Park, Young Joo Park, Shinje Moon
- Endocrinol Metab. 2024;39(1):140-151. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1748
- 1,015 View
- 52 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Many studies have shown that Hashimoto’s thyroiditis (HT) acts as a protective factor in differentiated thyroid cancer (DTC), but little is known about its effects on mortality. Therefore, this study was performed to reveal the prognosis of HT on mortality in patients with DTC.
Methods
This study included two types of research results: retrospective cohort study using the National Epidemiologic Survey of Thyroid cancer (NEST) in Korea and meta-analysis study with the NEST data and eight selected studies.
Results
Of the 4,398 patients with DTC in NEST, 341 patients (7.8%) died during the median follow-up period of 15 years (interquartile range, 12.3 to 15.6). Of these, 91 deaths (2.1%) were related to DTC. HT was associated with a smaller tumor size and less aggressive DTC. In Cox regression analysis after adjusting for age and sex, patients with HT showed a significantly lower risk of all-cause death (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.52 to 0.96) and DTC-related death (HR, 0.33; 95% CI, 0.14 to 0.77). The analysis with inverse probability of treatment weight data adjusted for age, sex, and year of thyroid cancer registration showed similar association. The meta-analysis showed that patients with HT showed a lower risk of all-cause mortality (risk ratio [RR], 0.24; 95% CI, 0.13 to 0.47) and thyroid cancer-related mortality (RR, 0.23; 95% CI, 0.13 to 0.40) in comparison with patients without HT.
Conclusion
This study showed that DTC co-presenting with HT is associated with a low risk of advanced DTC and presents a low risk for all-cause and DTC-related death.

Review Article
- Miscellaneous
- Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
- Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
- Endocrinol Metab. 2023;38(6):619-630. Published online November 21, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1814

- 2,491 View
- 111 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolism is a dynamic network of biochemical reactions that support systemic homeostasis amidst changing nutritional, environmental, and physical activity factors. The circulatory system facilitates metabolite exchange among organs, while the endocrine system finely tunes metabolism through hormone release. Endocrine disorders like obesity, diabetes, and Cushing’s syndrome disrupt this balance, contributing to systemic inflammation and global health burdens. They accompany metabolic changes on multiple levels from molecular interactions to individual organs to the whole body. Understanding how metabolic fluxes relate to endocrine disorders illuminates the underlying dysregulation. Cancer is increasingly considered a systemic disorder because it not only affects cells in localized tumors but also the whole body, especially in metastasis. In tumorigenesis, cancer-specific mutations and nutrient availability in the tumor microenvironment reprogram cellular metabolism to meet increased energy and biosynthesis needs. Cancer cachexia results in metabolic changes to other organs like muscle, adipose tissue, and liver. This review explores the interplay between the endocrine system and systems-level metabolism in health and disease. We highlight metabolic fluxes in conditions like obesity, diabetes, Cushing’s syndrome, and cancers. Recent advances in metabolomics, fluxomics, and systems biology promise new insights into dynamic metabolism, offering potential biomarkers, therapeutic targets, and personalized medicine.
-
Citations
Citations to this article as recorded by- Editorial: Tumor metabolism and programmed cell death
Dan-Lan Pu, Qi-Nan Wu
Frontiers in Endocrinology.2024;[Epub] CrossRef
- Editorial: Tumor metabolism and programmed cell death

Original Article
- Thyroid
- Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
- Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
- Endocrinol Metab. 2023;38(5):588-595. Published online September 8, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1723

- 1,490 View
- 91 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid cancer mortality has been largely overlooked as relatively stable given the large gap between thyroid cancer incidence and mortality. This study evaluated long-term trends in age-standardized mortality rates (ASMRs) throughout Korea and compared them with mortality data reported by the Surveillance, Epidemiology, and End Results (SEER).
Methods
Cancer-specific mortality data from 1985 to 2020 were obtained from Statistics Korea. ASMRs from thyroid cancer were calculated based on the Korean mid-year resident registration population of 2005. We assessed SEER*Explorer and downloaded the mortality data.
Results
The ASMR increased from 0.19 to 0.77/100,000 between 1985 and 2002 but decreased continuously to 0.36/100,000 in 2020. The annual percent change (APC) in the ASMR between 1985 and 2003 and between 2003 and 2020 was 6.204 and −4.218, respectively, with similar patterns observed in both men and women. The ASMR of the SEER showed a modest increase from 1988 to 2016 and then stabilized. In subgroup analysis, the ASMR of the old age group (≥55 years) increased significantly from 0.82 in 1985 to 3.92/100,000 in 2002 (APC 6.917) but then decreased again to 1.86/100,000 in 2020 (APC −4.136). ASMRs according to the age group in the SEER showed a relatively stable trend even in the elderly group.
Conclusion
The ASMR of thyroid cancer in Korea had increased from 1985 to 2002 but has since been steadily decreasing. This trend was mainly attributed to elderly people aged 55 or over. The absolute APC value of Korea was much higher than that of the SEER. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef - A Clinical Audit of Thyroid Hormonal Replacement After Total Thyroidectomy
Islam Mansy, Abdelfatah M Elsenosy, Eslam M Hassan, Mujtaba Zakria
Cureus.2023;[Epub] CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

Review Article
- Thyroid
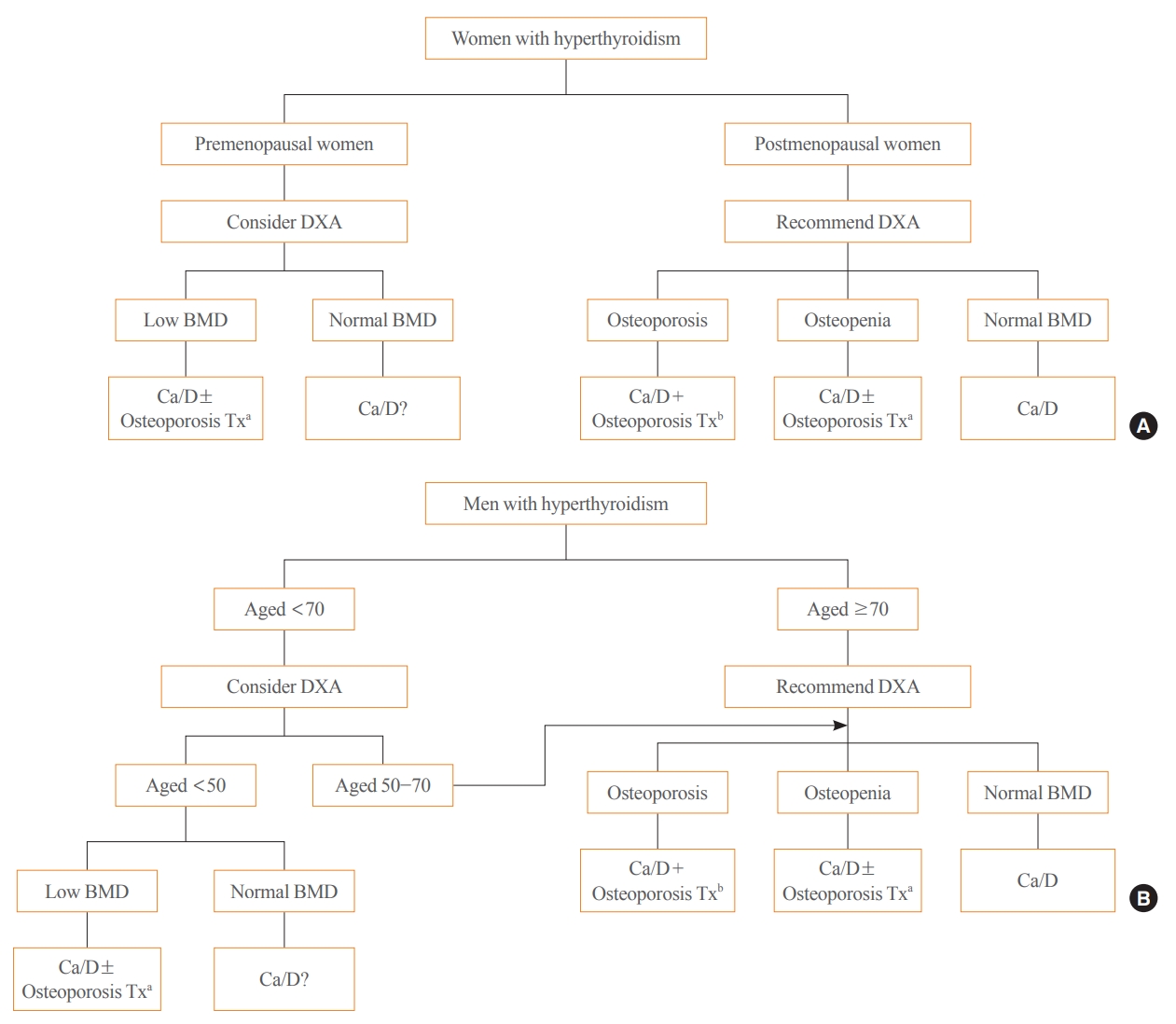
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
- A Ram Hong, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(2):175-189. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1701
- 3,959 View
- 248 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Thyroid hormones play an important physiological role in maintaining adult bone structure and strength. Consequently, thyroid dysfunction is related to skeletal outcomes. Overt hyperthyroidism is an established cause of high bone turnover with accelerated bone loss, leading to osteoporosis and increased fracture risk. Hyperthyroidism induced by thyroid-stimulating hormone-suppressive therapy in patients with differentiated thyroid cancer is a cause of secondary osteoporosis. In contrast, there is a lack of evidence on the negative impact of hypothyroidism on bone health. Considering the clinical updates on the importance of bone health in thyroid dysfunction, the Task Force from the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association recently developed a position statement on the evaluation and management of bone health of patients with thyroid diseases, particularly focused on endogenous hyperthyroidism and thyroid-stimulating hormone-suppressive therapy-associated hyperthyroidism in patients with differentiated thyroid cancer. Herein, we review the Korean Thyroid Association’s position statement on the evaluation and management of bone health associated with thyroid diseases.
-
Citations
Citations to this article as recorded by- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism
Justyna Kuliczkowska-Płaksej, Aleksandra Zdrojowy-Wełna, Aleksandra Jawiarczyk-Przybyłowska, Łukasz Gojny, Marek Bolanowski
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights
Shunichi Yokota, Hotaka Ishizu, Takuji Miyazaki, Daisuke Takahashi, Norimasa Iwasaki, Tomohiro Shimizu
Biomedicines.2024; 12(4): 843. CrossRef - Review on the protective activity of osthole against the pathogenesis of osteoporosis
Jincai Chen, Xiaofei Liao, Juwen Gan
Frontiers in Pharmacology.2023;[Epub] CrossRef
- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism

Original Articles
- Thyroid
Thyroid Cancer Screening - Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
- Leehi Joo, Min Kyoung Lee, Ji Ye Lee, Eun Ju Ha, Dong Gyu Na
- Endocrinol Metab. 2023;38(1):117-128. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1670

- 2,241 View
- 167 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study investigated the diagnostic performance of biopsy criteria in four society ultrasonography risk stratification systems (RSSs) for thyroid nodules, including the 2021 Korean (K)-Thyroid Imaging Reporting and Data System (TIRADS).
Methods
The Ovid-MEDLINE, Embase, Cochrane, and KoreaMed databases were searched and a manual search was conducted to identify original articles investigating the diagnostic performance of biopsy criteria for thyroid nodules (≥1 cm) in four widely used society RSSs.
Results
Eleven articles were included. The pooled sensitivity and specificity were 82% (95% confidence interval [CI], 74% to 87%) and 60% (95% CI, 52% to 67%) for the American College of Radiology (ACR)-TIRADS, 89% (95% CI, 85% to 93%) and 34% (95% CI, 26% to 42%) for the American Thyroid Association (ATA) system, 88% (95% CI, 81% to 92%) and 42% (95% CI, 22% to 67%) for the European (EU)-TIRADS, and 96% (95% CI, 94% to 97%) and 21% (95% CI, 17% to 25%) for the 2016 K-TIRADS. The sensitivity and specificity were 76% (95% CI, 74% to 79%) and 50% (95% CI, 49% to 52%) for the 2021 K-TIRADS1.5 (1.5-cm size cut-off for intermediate-suspicion nodules). The pooled unnecessary biopsy rates of the ACR-TIRADS, ATA system, EU-TIRADS, and 2016 K-TIRADS were 41% (95% CI, 32% to 49%), 65% (95% CI, 56% to 74%), 68% (95% CI, 60% to 75%), and 79% (95% CI, 74% to 83%), respectively. The unnecessary biopsy rate was 50% (95% CI, 47% to 53%) for the 2021 K-TIRADS1.5.
Conclusion
The unnecessary biopsy rate of the 2021 K-TIRADS1.5 was substantially lower than that of the 2016 K-TIRADS and comparable to that of the ACR-TIRADS. The 2021 K-TIRADS may help reduce potential harm due to unnecessary biopsies. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung
International Journal of Thyroidology.2023; 16(1): 1. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- To Screen or Not to Screen?

- Thyroid
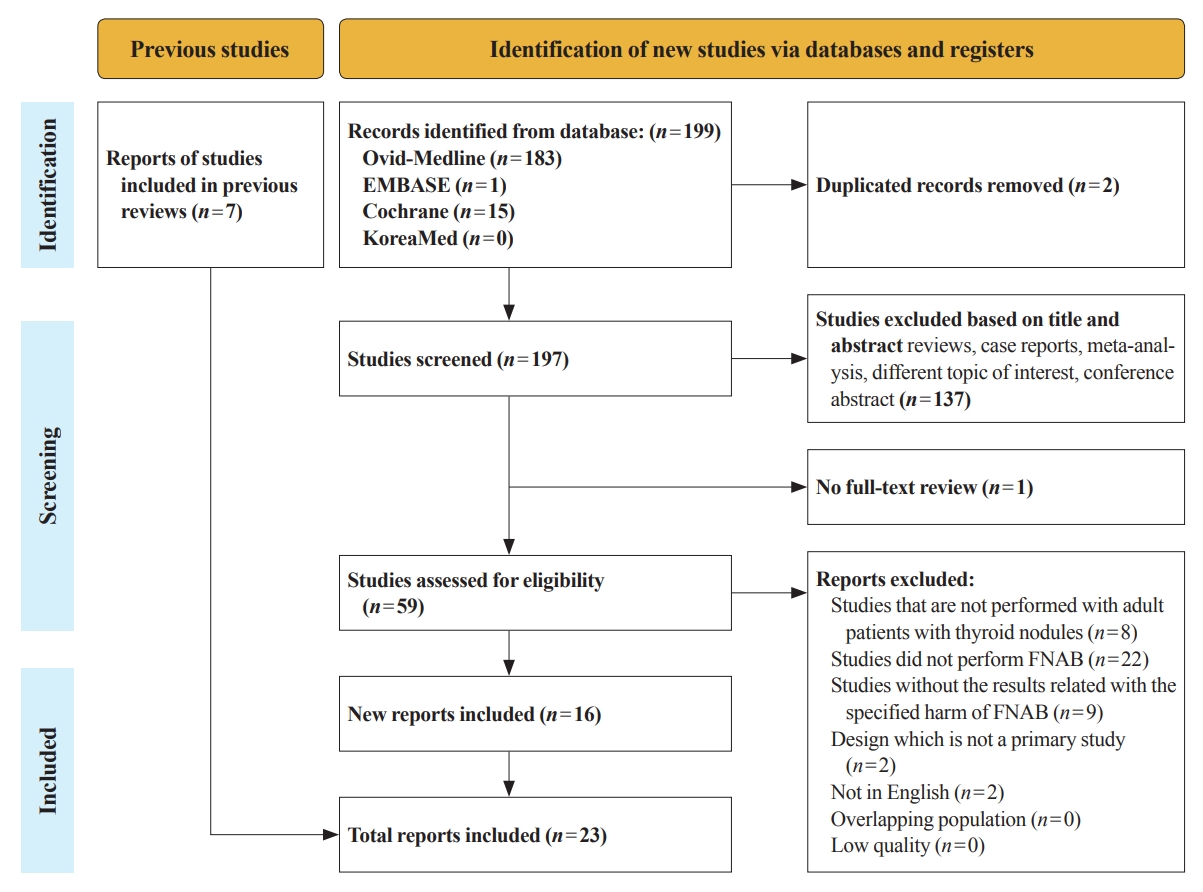
Thyroid Cancer Screening - A Comprehensive Assessment of the Harms of Fine-Needle Aspiration Biopsy for Thyroid Nodules: A Systematic Review
- Ji Yong Park, Wonsuk Choi, A Ram Hong, Jee Hee Yoon, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(1):104-116. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1669
- 3,763 View
- 162 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There have concerns related with the potential harms of fine-needle aspiration biopsy (FNAB). We aimed to summarize the clinical complications and evaluate the safety of FNAB.
Methods
Studies related with the harms of FNAB were searched on MEDLINE, Embase, Cochrane library, and KoreaMed from 2012 to 2022. Also, studies reviewed in the previous systematic reviews were evaluated. Included clinical complications were postprocedural pain, bleeding events, neurological symptoms, tracheal puncture, infections, post-FNAB thyrotoxicosis, and needle tract implantation of thyroid cancers.
Results
Twenty-three cohort studies were included in this review. Nine studies which were related with FNAB-related pain showed that most of the subjects had no or mild discomfort. The 0% to 6.4% of the patients had hematoma or hemorrhage after FNAB, according to 15 studies. Vasovagal reaction, vocal cord palsy, and tracheal puncture have rarely described in the included studies. Needle tract implantation of thyroid malignancies was described in three studies reporting 0.02% to 0.19% of the incidence rate.
Conclusion
FNAB is considered to be a safe diagnostic procedure with rare complications, which are mainly minor events. Thorough assessement of the patients’ medical condition when deciding to perform FNABs would be advisable to lower potential complications. -
Citations
Citations to this article as recorded by- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
Endocrinology and Metabolism.2024; 39(1): 61. CrossRef - Fine-needle aspiration cytology for neck lesions in patients with antithrombotic/anticoagulant medications: systematic review and meta-analysis
Dongbin Ahn, Ji Hye Kwak, Gill Joon Lee, Jin Ho Sohn
European Radiology.2024;[Epub] CrossRef - 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules

- Thyroid
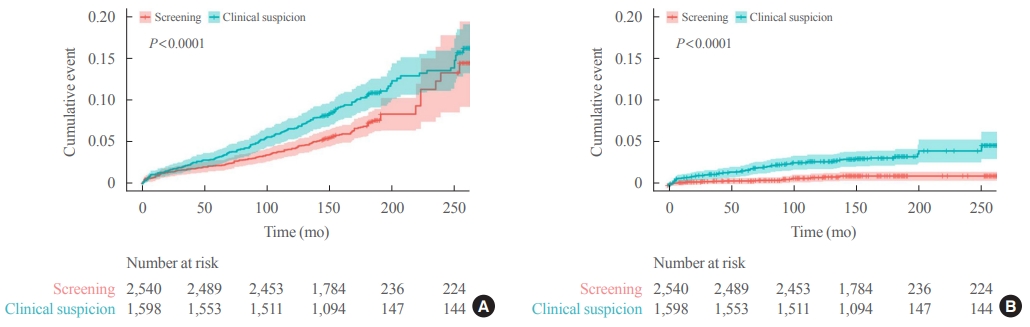
Thyroid Cancer Screening - Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
- Shinje Moon, Eun Kyung Lee, Hoonsung Choi, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2023;38(1):81-92. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1668
- 1,751 View
- 155 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The true benefit of thyroid cancer screening is incompletely understood. This study investigated the impact of ultrasound screening on thyroid cancer outcomes through a comparison with symptomatic thyroid cancer using data from a nationwide cohort study in Korea.
Methods
Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortality. Considering the possible bias arising from age, sex, year of thyroid cancer registration, and confounding factors for mortality (including smoking/drinking status, diabetes, and hypertension), all analyses were conducted with stabilized inverse probability of treatment weighting (IPTW) according to the route of detection.
Results
Of 5,796 patients with thyroid cancer, 4,145 were included and 1,651 were excluded due to insufficient data. In comparison with the screening group, the clinical suspicion group was associated with large tumors (17.2±14.6 mm vs. 10.4±7.9 mm), advanced T stage (3–4) (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.09 to 1.41), extrathyroidal extension (OR, 1.16; 95% CI, 1.02 to 1.32), and advanced stage (III–IV) (OR, 1.16; 95% CI, 1.00 to 1.35). In IPTW-adjusted Cox regression analysis, the clinical suspicion group had significantly higher risks of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29). Mediation analysis showed that the presence of thyroid-specific symptoms was directly associated with a higher risk of cancer-specific mortality. Thyroid-specific symptoms also indirectly affected thyroid cancer-specific mortality, mediated by tumor size and advanced clinicopathologic status.
Conclusion
Our findings provide important evidence for the survival benefit of early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
Endocrinology and Metabolism.2024; 39(2): 310. CrossRef - Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study
Tom Jansen, Nike Stikkelbroeck, Annenienke van de Ven, Ilse van Engen-van Grunsven, Marcel Janssen, Han Bonenkamp, Martin Gotthardt, Romana T. Netea-Maier
Cancers.2023; 15(8): 2350. CrossRef - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 93. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef
- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population

- Thyroid
Thyroid Cancer Screening - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
- Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
- Endocrinol Metab. 2023;38(1):93-103. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1667

- 2,205 View
- 119 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid cancer screening has contributed to the skyrocketing prevalence of thyroid cancer. However, the true benefit of thyroid cancer screening is not fully understood. This study aimed to evaluate the impact of screening on the clinical outcomes of thyroid cancer by comparing incidental thyroid cancer (ITC) with non-incidental thyroid cancer (NITC) through a meta-analysis.
Methods
PubMed and Embase were searched from inception to September 2022. We estimated and compared the prevalence of high-risk features (aggressive histology of thyroid cancer, extrathyroidal extension, metastasis to regional lymph nodes or distant organs, and advanced tumor-node-metastasis [TNM] stage), thyroid cancer-specific death, and recurrence in the ITC and NITC groups. We also calculated pooled risks and 95% confidence intervals (CIs) of the outcomes derived from these two groups.
Results
From 1,078 studies screened, 14 were included. In comparison to NITC, the ITC group had a lower incidence of aggressive histology (odds ratio [OR], 0.46; 95% CI, 0.31 to 0.7), smaller tumors (mean difference, −7.9 mm; 95% CI, −10.2 to −5.6), lymph node metastasis (OR, 0.64; 95% CI, 0.48 to 0.86), and distant metastasis (OR, 0.42; 95% CI, 0.23 to 0.77). The risks of recurrence and thyroid cancer-specific mortality were also lower in the ITC group (OR, 0.42; 95% CI, 0.25 to 0.71 and OR, 0.46; 95% CI, 0.28 to 0.74) than in the NITC group.
Conclusion
Our findings provide important evidence of a survival benefit from the early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Delayed Surgery for and Outcomes of Papillary Thyroid Cancer: Is the Pendulum Still Swinging?
Giorgio Grani
Clinical Thyroidology.2023; 35(5): 192. CrossRef
- To Screen or Not to Screen?

Review Article
- Thyroid
- Preclinical Models of Follicular Cell-Derived Thyroid Cancer: An Overview from Cancer Cell Lines to Mouse Models
- Min Ji Jeon, Bryan R. Haugen
- Endocrinol Metab. 2022;37(6):830-838. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1636

- 2,427 View
- 198 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The overall prognosis of thyroid cancer is excellent, but some patients have grossly invasive disease and distant metastases with limited responses to systemic therapies. Thus, relevant preclinical models are needed to investigate thyroid cancer biology and novel treatments. Different preclinical models have recently emerged with advances in thyroid cancer genetics, mouse modeling and new cell lines. Choosing the appropriate model according to the research question is crucial to studying thyroid cancer. This review will discuss the current preclinical models frequently used in thyroid cancer research, from cell lines to mouse models, and future perspectives on patient-derived and humanized preclinical models in this field.
-
Citations
Citations to this article as recorded by- Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication
Yue Ma, Bo Deng, Runbang He, Pengyu Huang
Heliyon.2024; 10(3): e24593. CrossRef - Strategies to investigate migration and metastases in thyroid cancer
Daniel M. Chopyk, Priya H. Dedhia
Current Opinion in Endocrine and Metabolic Research.2024; 34: 100502. CrossRef - Mouse Models to Examine Differentiated Thyroid Cancer Pathogenesis: Recent Updates
Hye Choi, Kwangsoon Kim
International Journal of Molecular Sciences.2023; 24(13): 11138. CrossRef - Modeling the tumor microenvironment of anaplastic thyroid cancer: an orthotopic tumor model in C57BL/6 mice
Zhen Xu, Hyo Shik Shin, Yoo Hyung Kim, Seong Yun Ha, Jae-Kyung Won, Su-jin Kim, Young Joo Park, Sareh Parangi, Sun Wook Cho, Kyu Eun Lee
Frontiers in Immunology.2023;[Epub] CrossRef - Patient-derived tumor models: a suitable tool for preclinical studies on esophageal cancer
Fan Liang, Hongyan Xu, Hongwei Cheng, Yabo Zhao, Junhe Zhang
Cancer Gene Therapy.2023; 30(11): 1443. CrossRef - Mechanistic Insights of Thyroid Cancer Progression
Luis Javier Leandro-García, Iñigo Landa
Endocrinology.2023;[Epub] CrossRef - Advances of Osteosarcoma Models for Drug Discovery and Precision Medicine
Linyun Tan, Yitian Wang, Xin Hu, Guifeng Du, Xiaodi Tang, Li Min
Biomolecules.2023; 13(9): 1362. CrossRef
- Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication

Original Articles
- Thyroid
- BRAFV600E Mutation Enhances Estrogen-Induced Metastatic Potential of Thyroid Cancer by Regulating the Expression of Estrogen Receptors
- Minjun Kim, Su-jin Kim, Seong Yun Ha, Zhen Xu, Youngjin Han, Hyeon-Gun Jee, Sun Wook Cho, Young Joo Park, Kyu Eun Lee
- Endocrinol Metab. 2022;37(6):879-890. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1563

- 2,822 View
- 216 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Cross-talk between mitogen-activated protein kinase and estrogen has been reported; however, the role of BRAFV600E in the estrogen responsiveness of thyroid cancer is unknown. We elucidated the effect of BRAFV600E on the estrogen-induced increase in metastatic potential in thyroid cancer.
Methods
Using a pair of cell lines, human thyroid cell lines which harbor wild type BRAF gene (Nthy/WT) and Nthy/BRAFV600E (Nthy/V600E), the expression of estrogen receptors (ERs) and estrogen-induced metastatic phenotypes were evaluated. Susceptibility to ERα- and ERβ-selective agents was evaluated to confirm differential ER expression. ESR expression was analyzed according to BRAFV600E status and age (≤50 years vs. >50 years) using The Cancer Genome Atlas (TCGA) data.
Results
Estradiol increased the ERα/ERβ expression ratio in Nthy/V600E, whereas the decreased ERα/ERβ expression ratio was found in Nthy/WT. BRAFV600E-mutated cell lines showed a higher E2-induced increase in metastatic potential, including migration, invasion, and anchorage-independent growth compared with Nthy/WT. An ERα antagonist significantly inhibited migration in Nthy/V600E cells, whereas an ERβ agonist was more effective in Nthy/WT. In the BRAFV600E group, ESR1/ESR2 ratio was significantly higher in younger age group (≤50 years) compared with older age group (>50 years) by TCGA data analysis.
Conclusion
Our data show that BRAFV600E mutation plays a crucial role in the estrogen responsiveness of thyroid cancer by regulating ER expression. Therefore, BRAFV600E might be used as a biomarker when deciding future hormone therapies based on estrogen signaling in thyroid cancer patients. -
Citations
Citations to this article as recorded by- The importance of protein domain mutations in cancer therapy
Kiran Kumar Chitluri, Isaac Arnold Emerson
Heliyon.2024; 10(6): e27655. CrossRef - Three cases of thyroid cancer in transgender female veterans receiving gender-affirming estrogen treatment
John D. Christensen, Hiba T. Basheer
Endocrine and Metabolic Science.2024; 15: 100177. CrossRef - Thyroid Cancer Prevalence, Risk Exposure, and Clinical Features Among Transgender Female Veterans
John David Christensen, Hiba T Basheer, Jose Joaquin Lado Abeal
Journal of the Endocrine Society.2024;[Epub] CrossRef - Association of DNA Promoter Methylation and BRAF Mutation in Thyroid Cancer
Farzana Jasmine, Briseis Aschebrook-Kilfoy, Mohammad M. Rahman, Garrett Zaagman, Raymon H. Grogan, Mohammed Kamal, Habibul Ahsan, Muhammad G. Kibriya
Current Oncology.2023; 30(3): 2978. CrossRef - Editorial: Recent advances in papillary thyroid carcinoma: Progression, treatment and survival predictors
Erivelto Martinho Volpi, Margarita Carmen Ramirez-Ortega, Jose Federico Carrillo
Frontiers in Endocrinology.2023;[Epub] CrossRef
- The importance of protein domain mutations in cancer therapy

- Thyroid
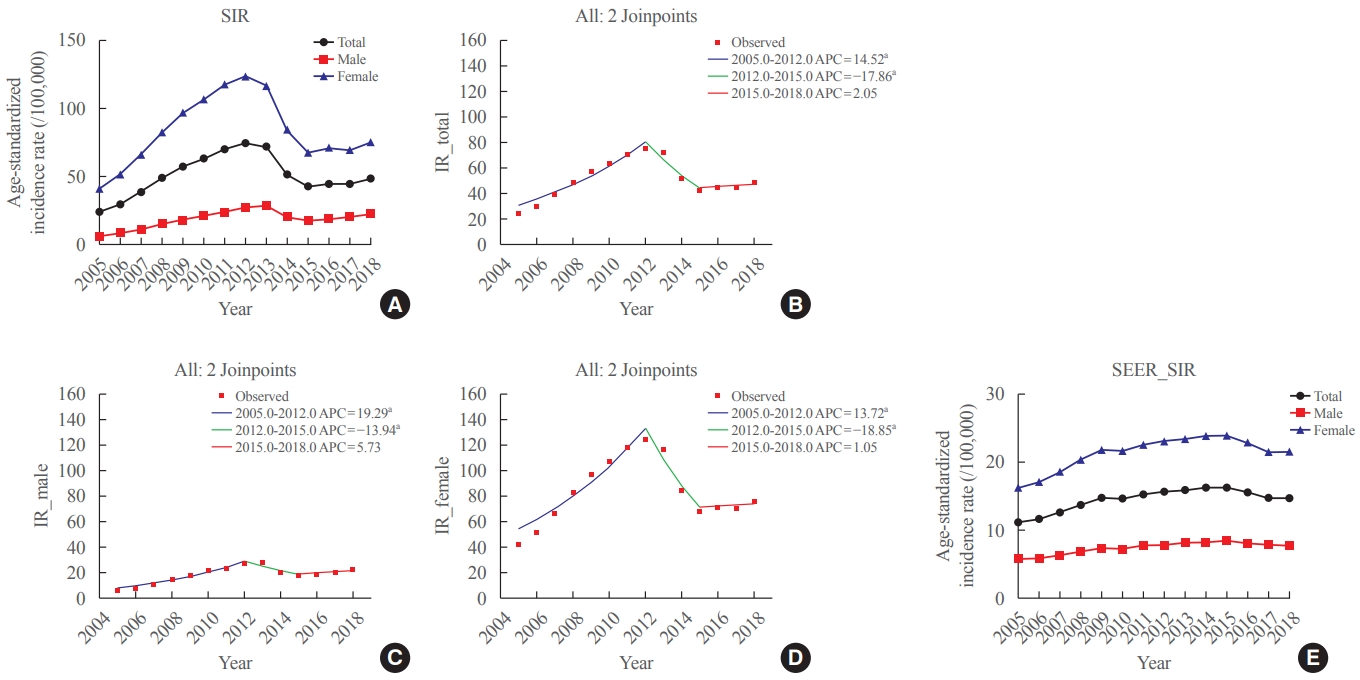
Big Data Articles (National Health Insurance Service Database) - Recent Changes in the Incidence of Thyroid Cancer in Korea between 2005 and 2018: Analysis of Korean National Data
- Yun Mi Choi, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
- Endocrinol Metab. 2022;37(5):791-799. Published online October 11, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1533
- 2,797 View
- 195 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
In this study, we evaluated the recent changes in the standardized, age-specific, stage-specific incidence rates (IRs) of thyroid cancer in Korea and compared them with the incidence data reported by the Surveillance, Epidemiology, and End Results Program.
Methods
The analysis was conducted using the incidence data (2005 to 2018) from the Statistics Korea and Korea Central Cancer Registry.
Results
The age-standardized IR (SIR) of thyroid cancer increased from 24.09 per 100,000 in 2005 to 74.83 in 2012 (annual percent change [APC], 14.5). From 2012 to 2015, the SIR decreased to 42.52 (APC, –17.9) and then remained stable until 2018 (APC, 2.1). This trend was similar in both men and women. Regarding age-specific IRs, the IRs for ages of 30 years and older showed a trend similar to that of the SIR; however, for ages below 30 years, no significant reduction was observed from the vertex of IR in 2015. Regarding stage-specific IRs, the increase was more prominent in those with regional disease (APC, 17.4) than in those with localized disease until 2012; then, the IR decreased until 2015 (APC, –16.1). The average APC from 2005 to 2018 increased in men, those under the age of 30 years, and those with regional disease.
Conclusion
The SIR in Korea peaked in 2012 and decreased until 2015 and then remained stable until 2018. However, in young individuals under the age of 30 years, the IR did not significantly decrease but tended to increase again. In terms of stage-specific IRs, the sharpest increase was seen among those with regional disease. -
Citations
Citations to this article as recorded by- Comparison of postoperative pain between transoral and conventional thyroidectomy: a propensity score-matched analysis
Min Kyu Park, Van Cuong Nguyen, Eugene Kim, Chang Myeon Song, Yong Bae Ji, Jin Hyeok Jeong, Kyung Tae
Surgical Endoscopy.2024; 38(3): 1512. CrossRef - Contents analysis of thyroid cancer-related information uploaded to YouTube by physicians in Korea: endorsing thyroid cancer screening, potentially leading to overdiagnosis
EunKyo Kang, HyoRim Ju, Soojeong Kim, Juyoung Choi
BMC Public Health.2024;[Epub] CrossRef - Bilateral axillo-breast approach robotic total thyroidectomy without isthmectomy: a case report
Hyeji Kim, Hyeonuk Hwang, Hyungju Kwon
The Ewha Medical Journal.2024;[Epub] CrossRef - Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study
Yu-Jin Kwon, Hye-Sun Lee, Sang-Wook Kang, Ji-Won Lee
Nutrients.2024; 16(7): 1041. CrossRef - Cancer and Mortality Risks of Graves’ Disease in South Korea Based on National Data from 2010 to 2019
Young Ju Choi, Kyungdo Han, Won Kyoung Cho, Min Ho Jung, Byung-Kyu Suh
Clinical Epidemiology.2023; Volume 15: 535. CrossRef - Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
Shinje Moon, Eun Kyung Lee, Hoonsung Choi, Sue K. Park, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 81. CrossRef - Cumulative exposure to metabolic syndrome increases thyroid cancer risk in young adults: a population-based cohort study
Jinyoung Kim, Kyungdo Han, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
The Korean Journal of Internal Medicine.2023; 38(4): 526. CrossRef - Cost-Effectiveness of Active Surveillance Compared to Early Surgery of Small Papillary Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Jaseong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chulmin Kim
Journal of Korean Medical Science.2023;[Epub] CrossRef - Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
Endocrinology and Metabolism.2023; 38(5): 588. CrossRef - Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients
Jin-Seong Cho, Yong-Min Na, Hee Kyung Kim
Cancers.2023; 15(23): 5506. CrossRef
- Comparison of postoperative pain between transoral and conventional thyroidectomy: a propensity score-matched analysis

Review Article
- Thyroid
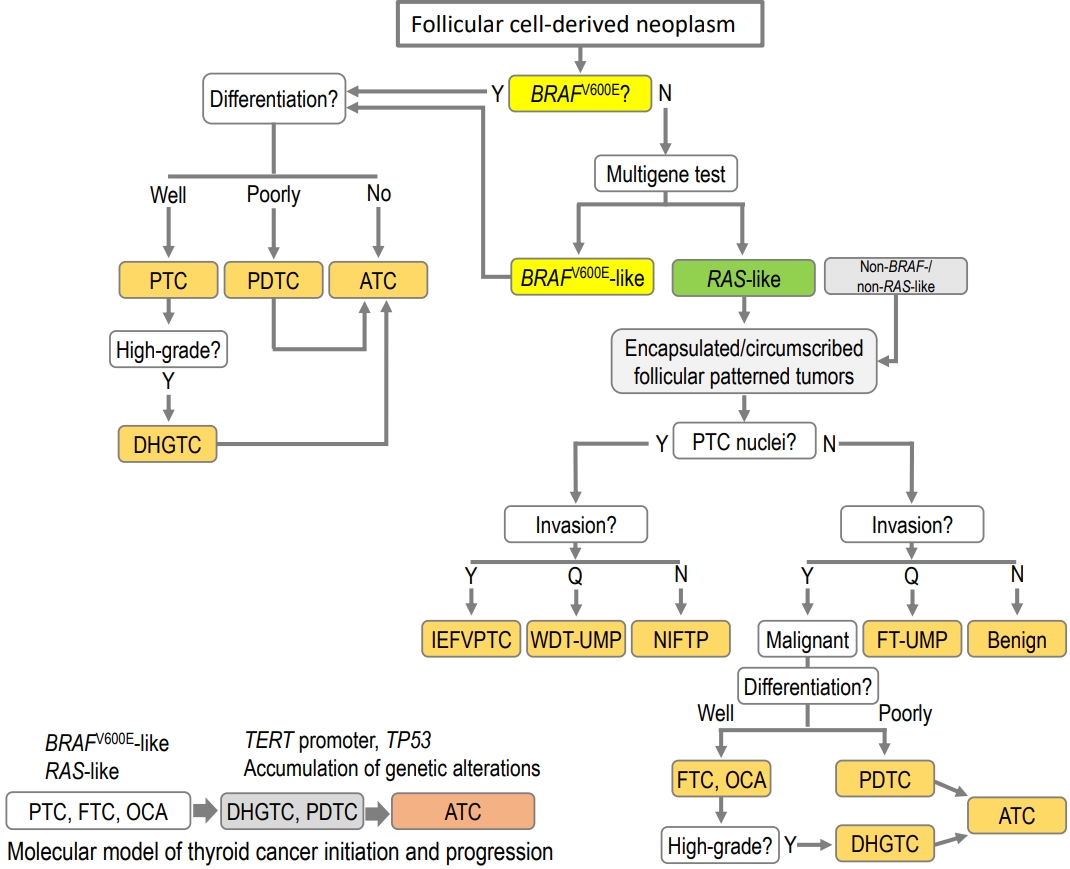
- Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
- Chan Kwon Jung, Andrey Bychkov, Kennichi Kakudo
- Endocrinol Metab. 2022;37(5):703-718. Published online October 4, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1553
- 16,576 View
- 2,122 Download
- 48 Web of Science
- 66 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The fifth edition of the World Health Organization (WHO) histologic classification of thyroid neoplasms released in 2022 includes newly recognized tumor types, subtypes, and a grading system. Follicular cell-derived neoplasms are categorized into three families (classes): benign tumors, low-risk neoplasms, and malignant neoplasms. The terms “follicular nodular disease” and “differentiated high-grade thyroid carcinoma” are introduced to account for multifocal hyperplastic/neoplastic lesions and differentiated thyroid carcinomas with high-grade features, respectively. The term “Hürthle cells” is replaced with “oncocytic cells.” Invasive encapsulated follicular and cribriform morular variants of papillary thyroid carcinoma (PTC) are now redefined as distinct tumor types, given their different genetic alterations and clinicopathologic characteristics from other PTC subtypes. The term “variant” to describe a subclass of tumor has been replaced with the term “subtype.” Instead, the term “variant” is reserved to describe genetic alterations. A histologic grading system based on the mitotic count, necrosis, and/or the Ki67 index is used to identify high-grade follicular-cell derived carcinomas and medullary thyroid carcinomas. The 2022 WHO classification introduces the following new categories: “salivary gland-type carcinomas of the thyroid” and “thyroid tumors of uncertain histogenesis.” This review summarizes the major changes in the 2022 WHO classification and their clinical relevance.
-
Citations
Citations to this article as recorded by- Tumeurs de la thyroïde : nouveautés de la classification OMS 2022
Serge Guyétant, Myriam Decaussin Petrucci, Emmanuelle Leteurtre
Annales de Pathologie.2024; 44(1): 5. CrossRef - Diagnostic Performance of [18F]TFB PET/CT Compared with Therapeutic Activity [131I]Iodine SPECT/CT and [18F]FDG PET/CT in Recurrent Differentiated Thyroid Carcinoma
David Ventura, Matthias Dittmann, Florian Büther, Michael Schäfers, Kambiz Rahbar, Daniel Hescheler, Michael Claesener, Philipp Schindler, Burkhard Riemann, Robert Seifert, Wolfgang Roll
Journal of Nuclear Medicine.2024; 65(2): 192. CrossRef - Clinicopathological features of differentiated thyroid carcinoma as predictors of the effects of radioactive iodine therapy
Wen Liu, Beibei Jiang, Jingli Xue, Ruijing Liu, Yuqing Wei, Peifeng Li
Annals of Diagnostic Pathology.2024; 69: 152243. CrossRef - Management of aggressive variants of papillary thyroid cancer
Ying Ki Lee, Aleix Rovira, Paul V. Carroll, Ricard Simo
Current Opinion in Otolaryngology & Head & Neck Surgery.2024; 32(2): 125. CrossRef - Artificial Intelligence Detected the Relationship Between Nuclear Morphological Features and Molecular Abnormalities of Papillary Thyroid Carcinoma
Toui Nishikawa, Ibu Matsuzaki, Ayata Takahashi, Iwamoto Ryuta, Fidele Yambayamba Musangile, Kanako Sagan, Mizuki Nishikawa, Yurina Mikasa, Yuichi Takahashi, Fumiyoshi Kojima, Shin-ichi Murata
Endocrine Pathology.2024; 35(1): 40. CrossRef - Review of the 2023 World Congress on Thyroid Cancer (WCTC 2023, London): is there light at the end of the tunnel for patients with neglected cancer?
S.M. Cherenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2024; 19(8): 609. CrossRef - Molecular Alterations and Comprehensive Clinical Management of Oncocytic Thyroid Carcinoma
Lindsay A. Bischoff, Ian Ganly, Laura Fugazzola, Erin Buczek, William C. Faquin, Bryan R. Haugen, Bryan McIver, Caitlin P. McMullen, Kate Newbold, Daniel J. Rocke, Marika D. Russell, Mabel Ryder, Peter M. Sadow, Eric Sherman, Maisie Shindo, David C. Shonk
JAMA Otolaryngology–Head & Neck Surgery.2024; 150(3): 265. CrossRef - Malignancy in a Solitary Thyroid Nodule: A Retrospective Histopathological Evaluation
Hassan Alzahrani
International Journal of General Medicine.2024; Volume 17: 135. CrossRef - Pathologie thyroïdienne. Actualités et problèmes pratiques. Introduction
Serge Guyétant
Annales de Pathologie.2024; 44(2): 90. CrossRef - Diagnostic implication of thyroid spherules for cytological diagnosis of thyroid nodules
Heeseung Sohn, Kennichi Kakudo, Chan Kwon Jung
Cytopathology.2024; 35(3): 383. CrossRef - Aggressive Primary Thyroid Mucoepidermoid Carcinoma with Extensive Pulmonary Involvement
Marius Mitrache, Dana Terzea, Anca Sirbu, Simona Fica
Biomedicines.2024; 12(2): 285. CrossRef - Unravelling the role of long non-coding RNAs in modulating the Hedgehog pathway in cancer
Shailendra Singh Chandel, Anurag Mishra, Gaurav Dubey, Ravindra Pal Singh, Mithilesh Singh, Mohit Agarwal, Himmat Singh Chawra, Neelima Kukreti
Pathology - Research and Practice.2024; 254: 155156. CrossRef - Hürthle cell (Oncocytic) carcinoma – Is hemithyroidectomy enough?
Taylor O. Julsrud, Trenton R. Foster, Robert A. Vierkant, Melanie L. Lyden, Travis J. McKenzie, Mabel Ryder, Benzon M. Dy
Surgical Oncology Insight.2024; 1(1): 100010. CrossRef - Échographie des carcinomes thyroïdiens différenciés de souche folliculaire
P.Y. Marcy, M. Tassart, J.G. Marchand, J. Sanglier, A. Bizeau, E. Ghanassia
Journal d'imagerie diagnostique et interventionnelle.2024; 7(2): 54. CrossRef - Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Chankyung Kim, Shipra Agarwal, Andrey Bychkov, Jen-Fan Hang, Agnes Stephanie Harahap, Mitsuyoshi Hirokawa, Kennichi Kakudo, Somboon Keelawat, Chih-Yi Liu, Zhiyan Liu, Truong Phan-Xuan Nguyen, Chanchal Rana, Huy Gia Vuong, Yun Zhu, Chan Kwon Jung
Virchows Archiv.2024;[Epub] CrossRef - Cas no5. High-grade Tall cell papillary carcinoma
Myriam Decaussin-Petrucci
Annales de Pathologie.2024; 44(2): 114. CrossRef - Current and future of immunotherapy for thyroid cancer based on bibliometrics and clinical trials
Ke Wang, Ying Zhang, Yang Xing, Hong Wang, Minghua He, Rui Guo
Discover Oncology.2024;[Epub] CrossRef - Cas no6. Anaplastic thyroid carcinoma, epidermoid subtype
Myriam Decaussin-Petrucci
Annales de Pathologie.2024; 44(2): 120. CrossRef - Papillary Thyroid Carcinoma With Lymphoepithelial Features and Lacking Association With Epstein-Barr Virus (EBV): A Rare Case
Ahmed Bendari, Saroja Devi Geetha, Reham Al-Refai, Xuelin Zhong, Sunder Sham, Manju Harshan
Cureus.2024;[Epub] CrossRef - Clinical and morphological features and unsolved issues in diagnosis of aggressive forms of papillary thyroid carcinoma
Denis V. Korotovsky, Sergey V. Sergiiko, Sergey A. Lukyanov
Perm Medical Journal.2024; 41(1): 90. CrossRef - Finding possible diagnostic markers for differentiating benign and malignant thyroid tumors on example investigate of rheological properties1
I. Javakhishvili, T. Sanikidze, K. Mardaleishvili, N. Momtselidze, T. Urdulashvili, M. Mantskava, L. Prantl, F. Jung
Clinical Hemorheology and Microcirculation.2024; : 1. CrossRef - Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma
Lia Rodrigues, Arnaud Da Cruz Paula, Paula Soares, João Vinagre
Cells.2024; 13(7): 561. CrossRef - Management of Poorly Differentiated Thyroid Cancer and Differentiated High-Grade Thyroid Carcinoma
Iram S. Alam, Kepal N. Patel
Surgical Clinics of North America.2024;[Epub] CrossRef - What’s new in thyroid pathology 2024: updates from the new WHO classification and bethesda system
Andrey Bychkov, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(2): 98. CrossRef - Ultrasound–Histopathological Presentation of Thyroid and Ovary Lesions in Adolescent Patients with DICER1 Syndrome: Case Reports and Literature Overview
Dominika Januś, Monika Kujdowicz, Konrad Kaleta, Kamil Możdżeń, Jan Radliński, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Marcin Maślanka, Wojciech Górecki, Jerzy B. Starzyk
Children.2024; 11(4): 403. CrossRef - Surgical treatment of solid variant of papillary thyroid carcinoma: Fifteen-year experience of a tertiary center
Katarina Tausanović, Marina Stojanović, Milan Jovanović, Boban Stepanović, Jovan Ilić, Sara Ivaniš, Vladan Živaljević
Medicinska istrazivanja.2024; 57(1): 121. CrossRef - Critical appraisal of the WHO 2022 classification of thyroid cancer
Mithraa Devi Sekar, Debasis Gochhait, Sadishkumar Kamalanathan
Thyroid Research and Practice.2024; 20(1): 8. CrossRef - Patterns in the Reporting of Aggressive Histologic Subtypes in Papillary Thyroid Cancer
Yeon J. Lee, Caitlin E. Egan, Jacques A. Greenberg, Teagan Marshall, Abhinay Tumati, Brendan M. Finnerty, Toni Beninato, Rasa Zarnegar, Thomas J. Fahey, Minerva A. Romero Arenas
Journal of Surgical Research.2024; 298: 325. CrossRef - An individualized protein-based prognostic model to stratify pediatric patients with papillary thyroid carcinoma
Zhihong Wang, He Wang, Yan Zhou, Lu Li, Mengge Lyu, Chunlong Wu, Tianen He, Lingling Tan, Yi Zhu, Tiannan Guo, Hongkun Wu, Hao Zhang, Yaoting Sun
Nature Communications.2024;[Epub] CrossRef - Update on C-Cell Neuroendocrine Neoplasm: Prognostic and Predictive Histopathologic and Molecular Features of Medullary Thyroid Carcinoma
Chan Kwon Jung, Shipra Agarwal, Jen-Fan Hang, Dong-Jun Lim, Andrey Bychkov, Ozgur Mete
Endocrine Pathology.2023; 34(1): 1. CrossRef - The 5th edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma
Fulvio Basolo, Elisabetta Macerola, Anello Marcello Poma, Liborio Torregrossa
Endocrine.2023; 80(3): 470. CrossRef - Preoperative Risk Stratification of Follicular-patterned Thyroid Lesions on Core Needle Biopsy by Histologic Subtyping and RAS Variant-specific Immunohistochemistry
Meejeong Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2023; 34(2): 247. CrossRef - Ultrasound evolution of parenchymal changes in the thyroid gland with autoimmune thyroiditis in children prior to the development of papillary thyroid carcinoma – a follow-up study
Dominika Januś, Monika Kujdowicz, Małgorzata Wójcik, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Wojciech Górecki, Jerzy B. Starzyk
Frontiers in Endocrinology.2023;[Epub] CrossRef - Papillary thyroid carcinoma with aggressive fused follicular and solid growth pattern: A unique histological subtype with high‐grade malignancy?
Shin‐ichi Murata, Ibu Matsuzaki, Mitsuo Kishimoto, Naomi Katsuki, Toshinori Onishi, Mitsuyoshi Hirokawa, Fumiyoshi Kojima
Pathology International.2023; 73(5): 207. CrossRef - Multi-Omics and Management of Follicular Carcinoma of the Thyroid
Thifhelimbilu Emmanuel Luvhengo, Ifongo Bombil, Arian Mokhtari, Maeyane Stephens Moeng, Demetra Demetriou, Claire Sanders, Zodwa Dlamini
Biomedicines.2023; 11(4): 1217. CrossRef - Molecular Genetics of Diffuse Sclerosing Papillary Thyroid Cancer
Meshael Alswailem, Balgees Alghamdi, Anwar Alotaibi, Abeer Aljomiah, Hindi Al-Hindi, Avaniyapuram Kannan Murugan, Mohamed Abouelhoda, Yufei Shi, Ali S Alzahrani
The Journal of Clinical Endocrinology & Metabolism.2023; 108(9): e704. CrossRef - Multifunctional Phase-Transition Nanoparticles for Effective Targeted Sonodynamic-Gene Therapy Against Thyroid Papillary Carcinoma
Shihui Guan, Dengke Teng, Hui Wang, Qimeihui Wang, Xi Zhen, Guoqing Sui, Yang Wang, Lingyu Zhu, Yuanqiang Lin, Dan Jiao, Feng Guo
International Journal of Nanomedicine.2023; Volume 18: 2275. CrossRef - Utilizing Dynamic Risk Stratification in Patients With Tall Cell Variant Papillary Thyroid Cancer
David Zimmer, Gilman Plitt, Brandon Prendes, Jamie Ku, Natalie Silver, Eric Lamarre, Emrullah Yilmaz, Jessica Geiger, Christian Nasr, Lea El Hage, Mario Skugor, Shauna Cambpell, Shlomo Koyfman, Jacob Miller, Neil Woody, Katherine Heiden, Nikhil Joshi, Tar
The Laryngoscope.2023; 133(9): 2430. CrossRef - Ultrasound, laboratory and histopathological insights in diagnosing papillary thyroid carcinoma in a paediatric population: a single centre follow-up study between 2000-2022
Dominika Januś, Małgorzata Wójcik, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Monika Kujdowicz, Małgorzata Czogała, Wojciech Górecki, Jerzy B. Starzyk
Frontiers in Endocrinology.2023;[Epub] CrossRef - Medullary Thyroid Carcinoma: A Single Institute Experience
Sonal Trivedi, T. Salahuddin, Mohamed Taher Mithi, Priyank Rathod, Arpit Bandi, Shashank J. Pandya, Mohit Sharma, Shailesh Patel, Vikas Warikoo, Ketul Puj, Abhijeet Salunkhe, Keval Patel, Shivam Pandya
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(4): 2884. CrossRef - FNAC (Fine needle aspiration cytology) and histopathological correlation and reclassification of thyroid neoplasm in accordance with WHO classification 2022
Fareeda Joshi, Shreya Hegde
Indian Journal of Pathology and Oncology.2023; 10(2): 132. CrossRef - Papillary thyroid carcinoma associated with non‑functioning parathyroid carcinoma with Warthin's tumor of the parotid gland: A case report and brief literature review
Ari Abdullah, Aras Qaradakhy, Yadgar Saeed, Abdulwahid Salih, Seema Karim, Osama Ali, Shko Hassan, Shalaw Nasraldeen, Shvan Mohammed, Fahmi Kakamad
Medicine International.2023;[Epub] CrossRef - Warthin-like papillary thyroid carcinoma: a case report and comprehensive review of the literature
Abdel Mouhaymen Missaoui, Fatma Hamza, Wafa Belabed, Manel Mellouli, Mohamed Maaloul, Slim Charfi, Issam Jardak, Tahya Sellami-Boudawara, Nabila Rekik, Mohamed Abid
Frontiers in Endocrinology.2023;[Epub] CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef - Cancer Predisposition Syndromes and Thyroid Cancer: Keys for a Short Two-Way Street
Ioana Balinisteanu, Monica-Cristina Panzaru, Lavinia Caba, Maria-Christina Ungureanu, Andreea Florea, Ana Maria Grigore, Eusebiu Vlad Gorduza
Biomedicines.2023; 11(8): 2143. CrossRef - Deep learning prediction model for central lymph node metastasis in papillary thyroid microcarcinoma based on cytology
Wenhao Ren, Yanli Zhu, Qian Wang, Yuntao Song, Zhihui Fan, Yanhua Bai, Dongmei Lin
Cancer Science.2023; 114(10): 4114. CrossRef - Cytology and Histology of Thyroid Nodules: Exploring Novel Insights in the Molecular Era for Enhanced Patient Management
Beatrix Cochand-Priollet, Zahra Maleki
Current Oncology.2023; 30(8): 7753. CrossRef - Agrin is a novel oncogenic protein in thyroid cancer
Anna Adamiok‑Ostrowska, Małgorzata Grzanka, Barbara Czarnocka
Oncology Letters.2023;[Epub] CrossRef - Analysis of Pathological Features of Thyroid Carcinoma in the Western Yunnan
身吾 王
Advances in Clinical Medicine.2023; 13(10): 15820. CrossRef - Insight of novel biomarkers for papillary thyroid carcinoma through multiomics
Wei Liu, Junkan Zhu, Zhen Wu, Yongxiang Yin, Qiao Wu, Yiming Wu, Jiaojiao Zheng, Cong Wang, Hongyan Chen, Talal Jamil Qazi, Jun Wu, Yuqing Zhang, Houbao Liu, Jingmin Yang, Daru Lu, Xumin Zhang, Zhilong Ai
Frontiers in Oncology.2023;[Epub] CrossRef - Identification of NIFTP-Specific mRNA Markers for Reliable Molecular Diagnosis of Thyroid Tumors
So-Yeon Lee, Jong-Lyul Park, Kwangsoon Kim, Ja Seong Bae, Jae-Yoon Kim, Seon-Young Kim, Chan Kwon Jung
Endocrine Pathology.2023; 34(3): 311. CrossRef - Somatostatin Receptor Type 2 and Thyroid-Stimulating Hormone Receptor Expression in Oncocytic Thyroid Neoplasms: Implications for Prognosis and Treatment
Andrea Gillis, Rui Zheng-Pywell, Chandler McLeod, Dezhi Wang, John M. Ness, Rachael Guenter, Jason Whitt, Tomas A. Prolla, Herbert Chen, Manuel Lora Gonzalez, Bart Rose, Ricardo V. Lloyd, Renata Jaskula-Sztul, Diana Lin
Modern Pathology.2023; 36(12): 100332. CrossRef - Comparison of Treatment and Prognosis Between Follicular Variant
Papillary Thyroid Carcinoma and Classical Papillary Thyroid
Carcinoma
Bing Zhang, Wenming Wu, Jinjing Liu, Zhou Liang, Liang Zong
Hormone and Metabolic Research.2023; 55(12): 855. CrossRef - Sentinel lymph node mapping: current applications and future perspectives in thyroid carcinoma
Isabella Merante Boschin, Loris Bertazza, Carla Scaroni, Caterina Mian, Maria Rosa Pelizzo
Frontiers in Medicine.2023;[Epub] CrossRef - Utility of the Growth Differentiation Factor-15 in the Differential Diagnosis of Follicular-Patterned Lesions of the Thyroid on Cytopathologic and Histopathologic Samples
Prasanna V Perumal, Neelaiah Siddaraju, Sunil K Saxena, Soundravally Rajendiran, Ramachandra V Bhat
Cureus.2023;[Epub] CrossRef - Investigation of pre‐operative demographic, biochemical, sonographic and cytopathological findings in low‐risk thyroid neoplasms
Muzaffer Serdar Deniz, Didem Özdemir, Narin Nasıroğlu İmga, Hüsniye Başer, Fatma Neslihan Çuhacı Seyrek, Ayşegül Aksoy Altınboğa, Oya Topaloğlu, Reyhan Ersoy, Bekir Çakır
Clinical Endocrinology.2023; 99(5): 502. CrossRef - Applications of machine and deep learning to thyroid cytology and histopathology: a review
Greg Slabaugh, Luis Beltran, Hasan Rizvi, Panos Deloukas, Eirini Marouli
Frontiers in Oncology.2023;[Epub] CrossRef - The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
Journal of Pathology and Translational Medicine.2023; 57(6): 289. CrossRef - Anaplastic thyroid cancer: Pathogenesis, prognostic factors and genetic landscape (Review)
Abdul-Mohsen Alhejaily, Omar Alhuzim, Yazeed Alwelaie
Molecular and Clinical Oncology.2023;[Epub] CrossRef - Simultaneous Occurrence of Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma: A Case Series with Literature Review
Poupak Fallahi, Armando Patrizio, Giulio Stoppini, Giusy Elia, Francesca Ragusa, Sabrina Rosaria Paparo, Eugenia Balestri, Valeria Mazzi, Chiara Botrini, Gilda Varricchi, Salvatore Ulisse, Marco Ghionzoli, Alessandro Antonelli, Silvia Martina Ferrari
Current Oncology.2023; 30(12): 10237. CrossRef - Construction and validation of a nomogram for predicting cervical lymph node metastasis in diffuse sclerosing variant of papillary thyroid carcinoma
Xunyi Lin, Jiaxing Huo, Huan Zhang, Hang Su, Fenghua Zhang
Langenbeck's Archives of Surgery.2023;[Epub] CrossRef - Canine follicular cell and medullary thyroid carcinomas: Immunohistochemical characterization
Jana Jankovic, Eve Tièche, Martina Dettwiler, Kerstin Hahn, Stephanie Scheemaeker, Martin Kessler, Sylvie Daminet, Sven Rottenberg, Miguel Campos
Veterinary Pathology.2023;[Epub] CrossRef - A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma
Julia I. Staubitz-Vernazza, Sina Schwind, Oana Lozan, Thomas J. Musholt
Cancers.2023; 16(1): 163. CrossRef - Reprogramming of Cellular Metabolism and Its Therapeutic Applications in Thyroid Cancer
Yuji Nagayama, Koichiro Hamada
Metabolites.2022; 12(12): 1214. CrossRef - Developments to improve outcomes in thyroid surgery
Thomas J. Musholt
Innovative Surgical Sciences.2022; 7(3-4): 77. CrossRef - The relationship of the clinicopathological characteristics and treatment results of post-Chornobyl papillary thyroid microcarcinomas with the latency period and radiation exposure
Tetiana Bogdanova, Serhii Chernyshov, Liudmyla Zurnadzhy, Tatiana I. Rogounovitch, Norisato Mitsutake, Mykola Tronko, Masahiro Ito, Michael Bolgov, Sergii Masiuk, Shunichi Yamashita, Vladimir A. Saenko
Frontiers in Endocrinology.2022;[Epub] CrossRef
- Tumeurs de la thyroïde : nouveautés de la classification OMS 2022


 KES
KES

 First
First Prev
Prev



