Search
- Page Path
- HOME > Search
Original Article
- Thyroid
- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
- Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
- Endocrinol Metab. 2024;39(2):310-323. Published online April 9, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1870
- 357 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is debate about ultrasonography screening for thyroid cancer and its cost-effectiveness. This study aimed to evaluate the cost-effectiveness of early screening (ES) versus symptomatic detection (SD) for differentiated thyroid cancer (DTC) in Korea.
Methods
A Markov decision analysis model was constructed to compare the cost-effectiveness of ES and SD. The model considered direct medical costs, health outcomes, and different diagnostic and treatment pathways. Input data were derived from literature and Korean population studies. Incremental cost-effectiveness ratio (ICER) was calculated. Willingness-to-pay (WTP) threshold was set at USD 100,000 or 20,000 per quality-adjusted life year (QALY) gained. Sensitivity analyses were conducted to address uncertainties of the model’s variables.
Results
In a base case scenario with 50 years of follow-up, ES was found to be cost-effective compared to SD, with an ICER of $2,852 per QALY. With WTP set at $100,000, in the case with follow-up less than 10 years, the SD was cost-effective. Sensitivity analysis showed that variables such as lobectomy probability, age, mortality, and utility scores significantly influenced the ICER. Despite variations in costs and other factors, all ICER values remained below the WTP threshold.
Conclusion
Findings of this study indicate that ES is a cost-effective strategy for DTC screening in the Korean medical system. Early detection and subsequent lobectomy contribute to the cost-effectiveness of ES, while SD at an advanced stage makes ES more cost-effective. Expected follow-up duration should be considered to determine an optimal strategy for DTC screening.

Review Article
- Calcium & bone metabolism
- Treatment of Hypoparathyroidism by Re-Establishing the Effects of Parathyroid Hormone
- Lars Rejnmark
- Endocrinol Metab. 2024;39(2):262-266. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1916
- 379 View
- 48 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The conventional treatment of hypoparathyroidism (HypoPT) includes active vitamin D and calcium. Despite normalization of calcium levels, the conventional treatment is associated with fluctuations in calcium levels, hypercalciuria, renal impairment, and decreased quality of life (QoL). Replacement therapy with parathyroid hormone (PTH)(1-84) is an option in some countries. However, convincing beneficial effects have not been demonstrated, which may be due to the short duration of action of this treatment. Recently, palopegteriparatide (also known as TransCon PTH) has been marketed in Europe and is expected also to be approved in other countries. Palopegteriparatide is a prodrug with sustained release of PTH(1-34) designed to provide stable physiological PTH levels for 24 hours/day. A phase 3 study demonstrated maintenance of normocalcemia in patients with chronic HypoPT, with no need for conventional therapy. Furthermore, this treatment lowers urinary calcium and improves QoL. Another long-acting PTH analog with effects on the parathyroid hormone receptor (eneboparatide) is currently being tested in a phase 3 trial. Furthermore, the treatment of autosomal dominant hypocalcemia type 1 with a calcilytic (encaleret) is also being tested. All in all, improved treatment options are on the way that will likely take the treatment of HypoPT to the next level.

Original Articles
- Thyroid
- Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
- Jinsun Lim, Han Sai Lee, Jin-Hyung Heo, Young Shin Song
- Endocrinol Metab. 2024;39(2):324-333. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1885
- 354 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The predictive factors for lateral neck lymph node metastasis (LLNM) in papillary thyroid microcarcinoma (PTMC) remain undetermined. This study investigated the clinicopathological characteristics, transcriptomes, and tumor microenvironment in PTMC according to the LLNM status. We aimed to identify the biomarkers associated with LLNM development.
Methods
We retrospectively reviewed the medical records of patients with PTMC from two independent institutions between 2018 and 2022 (n=597 and n=467). We compared clinicopathological features between patients without lymph node metastasis (N0) and those with LLNM (N1b). Additionally, laser capture microdissection and RNA sequencing were performed on primary tumors from both groups, including metastatic lymph nodes from the N1b group (n=30; 20 primary tumors and 10 paired LLNMs). We corroborated the findings using RNA sequencing data from 16 BRAF-like PTMCs from The Cancer Genome Atlas. Transcriptomic analyses were validated by immunohistochemical staining.
Results
Clinicopathological characteristics, such as male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis showed associations with LLNM in PTMCs. Transcriptomic profiles between the N0 and N1b PTMC groups were similar. However, tumor microenvironment deconvolution from RNA sequencing and immunohistochemistry revealed an increased abundance of tumor-associated macrophages, particularly M2 macrophages, in the N1b group.
Conclusion
Patients with PTMC who have a male sex, multifocality, extrathyroidal extension, lymphatic invasion, and central node metastasis exhibited an elevated risk for LLNM. Furthermore, infiltration of M2 macrophages in the tumor microenvironment potentially supports tumor progression and LLNM in PTMCs.

- Thyroid
- Prognostic Roles of Inflammatory Biomarkers in Radioiodine-Refractory Thyroid Cancer Treated with Lenvatinib
- Chae A Kim, Mijin Kim, Meihua Jin, Hee Kyung Kim, Min Ji Jeon, Dong Jun Lim, Bo Hyun Kim, Ho-Cheol Kang, Won Bae Kim, Dong Yeob Shin, Won Gu Kim
- Endocrinol Metab. 2024;39(2):334-343. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1854
- 308 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), serve as valuable prognostic indicators in various cancers. This multicenter, retrospective cohort study assessed the treatment outcomes of lenvatinib in 71 patients with radioactive iodine (RAI)-refractory thyroid cancer, considering the baseline inflammatory biomarkers.
Methods
This study retrospectively included patients from five tertiary hospitals in Korea whose complete blood counts were available before lenvatinib treatment. Progression-free survival (PFS) and overall survival (OS) were evaluated based on the median value of inflammatory biomarkers.
Results
No significant differences in baseline characteristics were observed among patients grouped according to the inflammatory biomarkers, except for older patients with a higher-than-median NLR (≥2) compared to their counterparts with a lower NLR (P= 0.01). Patients with a higher-than-median NLR had significantly shorter PFS (P=0.02) and OS (P=0.017) than those with a lower NLR. In multivariate analysis, a higher-than-median NLR was significantly associated with poor OS (hazard ratio, 3.0; 95% confidence interval, 1.24 to 7.29; P=0.015). However, neither the LMR nor the PLR was associated with PFS. A higher-than-median LMR (≥3.9) was significantly associated with prolonged OS compared to a lower LMR (P=0.036). In contrast, a higher-than-median PLR (≥142.1) was associated with shorter OS compared to a lower PLR (P=0.039).
Conclusion
Baseline inflammatory biomarkers can serve as predictive indicators of PFS and OS in patients with RAI-refractory thyroid cancer treated with lenvatinib.

- Protein Signatures of Parathyroid Adenoma according to Tumor Volume and Functionality
- Sung Hye Kong, Jeong Mo Bae, Jung Hee Kim, Sang Wan Kim, Dohyun Han, Chan Soo Shin
- Received September 14, 2023 Accepted December 21, 2023 Published online March 21, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1827 [Epub ahead of print]
- 387 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Parathyroid adenoma (PA) is a common endocrine disease linked to multiple complications, but the pathophysiology of the disease remains incompletely understood. The study aimed to identify the key regulator proteins and pathways of PA according to functionality and volume through quantitative proteomic analyses.
Methods
We conducted a retrospective study of 15 formalin-fixed, paraffin-embedded PA samples from tertiary hospitals in South Korea. Proteins were extracted, digested, and the resulting peptides were analyzed using liquid chromatography-tandem mass spectrometry. Pearson correlation analysis was employed to identify proteins significantly correlated with clinical variables. Canonical pathways and transcription factors were analyzed using Ingenuity Pathway Analysis.
Results
The median age of the participants was 52 years, and 60.0% were female. Among the 8,153 protein groups analyzed, 496 showed significant positive correlations with adenoma volume, while 431 proteins were significantly correlated with parathyroid hormone (PTH) levels. The proteins SLC12A9, LGALS3, and CARM1 were positively correlated with adenoma volume, while HSP90AB2P, HLA-DRA, and SCD5 showed negative correlations. DCPS, IRF2BPL, and FAM98A were the main proteins that exhibited positive correlations with PTH levels, and SLITRK4, LAP3, and AP4E1 had negative correlations. Canonical pathway analysis demonstrated that the RAN and sirtuin signaling pathways were positively correlated with both PTH levels and adenoma volume, while epithelial adherence junction pathways had negative correlations.
Conclusion
Our study identified pivotal proteins and pathways associated with PA, offering potential therapeutic targets. These findings accentuate the importance of proteomics in understanding disease pathophysiology and the need for further research.

- Thyroid
- Risk of Subsequent Primary Cancers in Thyroid Cancer Survivors according to the Dose of Levothyroxine: A Nationwide Cohort Study
- Min-Su Kim, Jang Won Lee, Min Kyung Hyun, Young Shin Song
- Endocrinol Metab. 2024;39(2):288-299. Published online March 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1815
- 642 View
- 36 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Current research has not investigated the effect of thyroid-stimulating hormone suppression therapy with levothyroxine on the risk for developing subsequent primary cancers (SPCs). This study aimed to investigate the association between levothyroxine dosage and the risk for SPCs in thyroid cancer patients.
Methods
We conducted a nationwide population-based retrospective cohort study form Korean National Health Insurance database. This cohort included 342,920 thyroid cancer patients between 2004 and 2018. Patients were divided into the non-levothyroxine and the levothyroxine groups, the latter consisting of four dosage subgroups according to quartiles. Cox proportional hazard models were performed to evaluate the risk for SPCs by adjusting for variables including cumulative doses of radioactive iodine (RAI) therapy.
Results
A total of 17,410 SPC cases were observed over a median 7.3 years of follow-up. The high-dose levothyroxine subgroups (Q3 and Q4) had a higher risk for SPC (adjusted hazard ratio [HR], 1.14 and 1.27; 95% confidence interval [CI], 1.05–1.24 and 1.17– 1.37; respectively) compared to the non-levothyroxine group. In particular, the adjusted HR of stomach (1.31), colorectal (1.60), liver and biliary tract (1.95), and pancreatic (2.48) cancers were increased in the Q4 subgroup. We consistently observed a positive association between high levothyroxine dosage per body weight and risk of SPCs, even after adjusting for various confounding variables. Moreover, similar results were identified in the stratified analyses according to thyroidectomy type and RAI therapy, as well as in a subgroup analysis of patients with good adherence.
Conclusion
High-dose levothyroxine use was associated with increased risk of SPCs among thyroid cancer patients regardless of RAI therapy.

Review Articles
- Thyroid
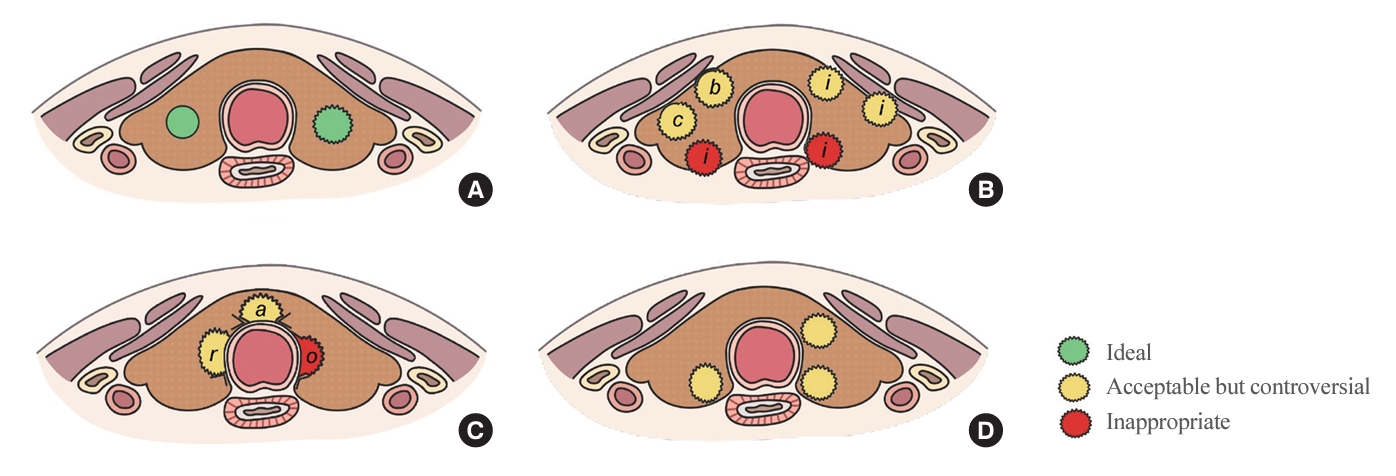
- Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Min Joo Kim, Jae Hoon Moon, Eun Kyung Lee, Young Shin Song, Kyong Yeun Jung, Ji Ye Lee, Ji-hoon Kim, Kyungsik Kim, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2024;39(1):47-60. Published online February 15, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1937
- 1,879 View
- 169 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.

- Thyroid
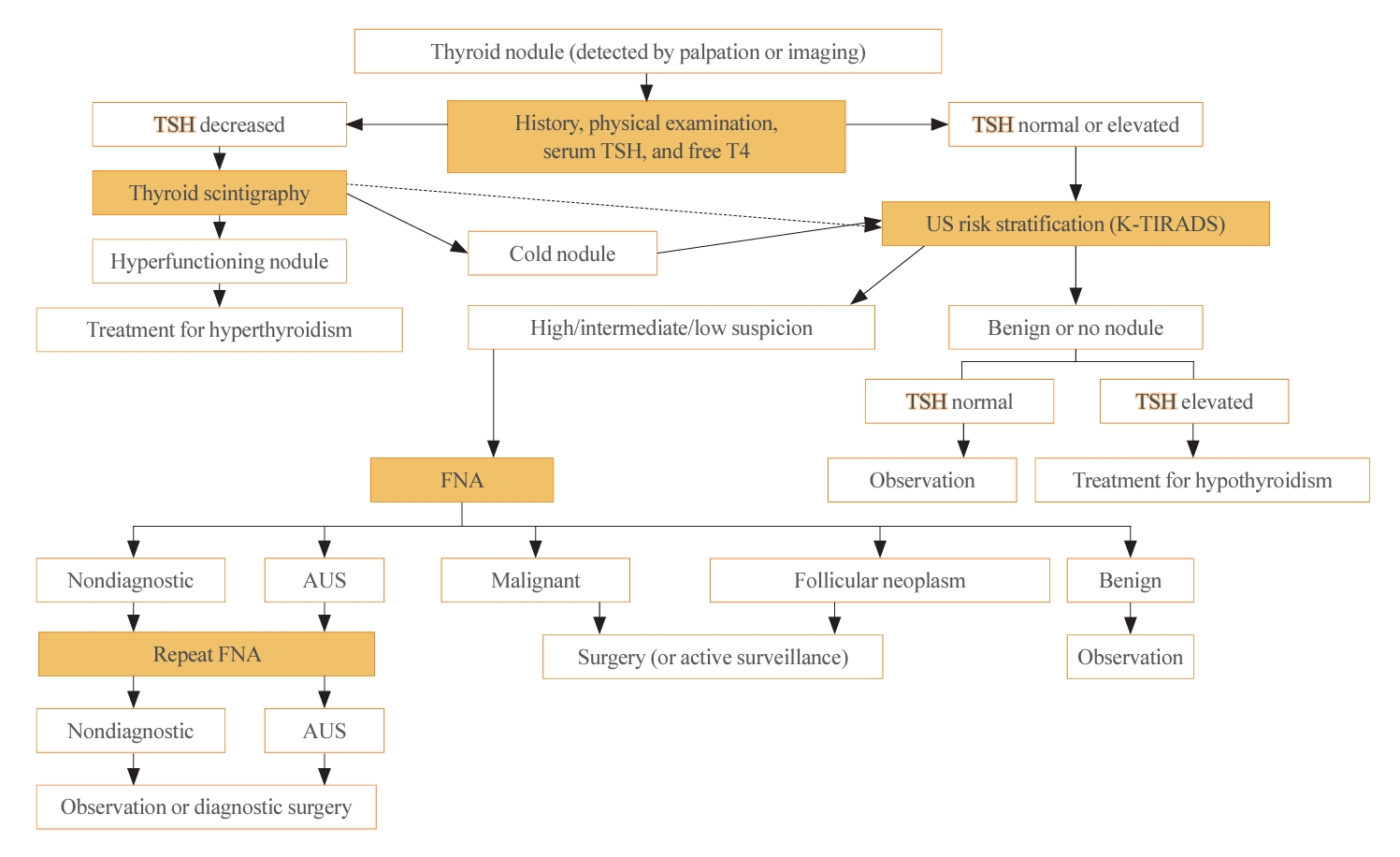
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
- Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
- Endocrinol Metab. 2024;39(1):61-72. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1938
- 1,484 View
- 92 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The 2023 Korean Thyroid Association (KTA) Management Guideline for Patients with Thyroid Nodules constitute an update of the 2016 KTA guideline for thyroid nodules and cancers that focuses specifically on nodules. The 2023 guideline aim to offer updated guidance based on new evidence that reflects the changes in clinical practice since the 2016 KTA guideline. To update the 2023 guideline, a comprehensive literature search was conducted from January 2022 to May 2022. The literature search included studies, reviews, and other evidence involving human subjects that were published in English in MEDLINE (PubMed), Embase, and other relevant databases. Additional significant clinical trials and research studies published up to April 2023 were also reviewed. The limitations of the current evidence are discussed, and suggestions for areas in need of further research are identified. The purpose of this review is to provide a summary of the 2023 KTA guideline for the management of thyroid nodules released in May 2023 and to give a balanced insight with comparison of recent guidelines from other societies.

- Thyroid
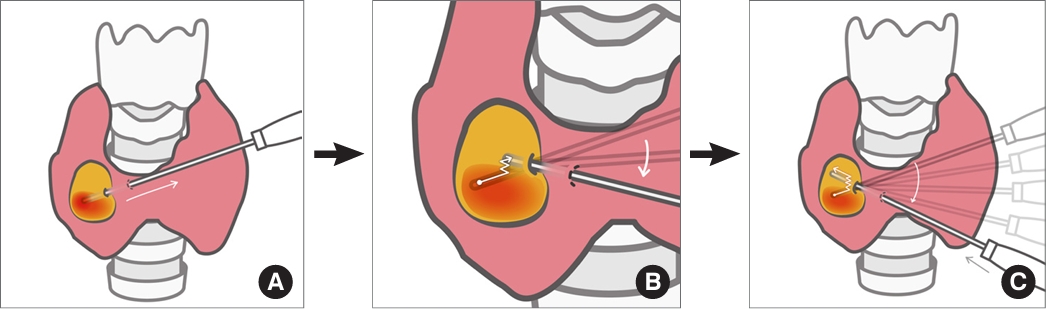
- Novel and Advanced Ultrasound Techniques for Thyroid Thermal Ablation
- Wai-Kin Chan, Jui-Hung Sun, Miaw-Jene Liou, Chia-Jung Hsu, Yu-Ling Lu, Wei-Yu Chou, Yan-Rong Li, Feng-Hsuan Liu
- Endocrinol Metab. 2024;39(1):40-46. Published online February 13, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1917
- 1,688 View
- 107 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Thyroid radiofrequency ablation and microwave ablation are widely adopted minimally invasive treatments for diverse thyroid conditions worldwide. Fundamental skills such as the trans-isthmic approach and the moving shot technique are crucial for performing thyroid ablation, and advanced techniques, including hydrodissection and vascular ablation, improve safety and efficacy and reduce complications. Given the learning curve associated with ultrasound-guided therapeutic procedures, operators need training and experience. While training models exist, limited attention has been given to ultrasound maneuvers in ablation needle manipulation. This article introduces two essential maneuvers, the zigzag moving technique and the alienate maneuver, while also reviewing the latest ultrasound techniques in thyroid ablation, contributing valuable insights into this evolving field.

Original Articles
- Clinical Characteristics, Diagnosis, and Treatment of Thyroid Stimulating Hormone-Secreting Pituitary Neuroendocrine Tumor (TSH PitNET): A Single-Center Experience
- Jung Heo, Yeon-Lim Suh, Se Hoon Kim, Doo-Sik Kong, Do-Hyun Nam, Won-Jae Lee, Sung Tae Kim, Sang Duk Hong, Sujin Ryu, You-Bin Lee, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim, Kyu Yeon Hur
- Received November 8, 2023 Accepted December 21, 2023 Published online February 5, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1877 [Epub ahead of print]
- 792 View
- 31 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid-stimulating hormone (TSH)-secreting pituitary neuroendocrine tumor (TSH PitNET) is a rare subtype of PitNET. We investigated the comprehensive characteristics and outcomes of TSH PitNET cases from a single medical center. Also, we compared diagnostic methods to determine which showed superior sensitivity.
Methods
A total of 17 patients diagnosed with TSH PitNET after surgery between 2002 and 2022 in Samsung Medical Center was retrospectively reviewed. Data on comprehensive characteristics and treatment outcomes were collected. The sensitivities of diagnostic methods were compared.
Results
Seven were male (41%), and the median age at diagnosis was 42 years (range, 21 to 65); the median follow-up duration was 37.4 months. The most common (59%) initial presentation was hyperthyroidism-related symptoms. Hormonal co-secretion was present in four (23%) patients. Elevated serum alpha-subunit (α-SU) showed the greatest diagnostic sensitivity (91%), followed by blunted response at thyrotropin-releasing hormone (TRH) stimulation (80%) and elevated sex hormone binding globulin (63%). Fourteen (82%) patients had macroadenoma, and a specimen of one patient with heavy calcification was negative for TSH. Among 15 patients who were followed up for more than 6 months, 10 (67%) achieved hormonal and structural remission within 6 months postoperatively. A case of growth hormone (GH)/TSH/prolactin (PRL) co-secreting mixed gangliocytoma-pituitary adenoma (MGPA) was discovered.
Conclusion
The majority of the TSH PitNET cases was macroadenoma, and 23% showed hormone co-secretion. A rare case of GH/TSH/PRL co-secreting MGPA was discovered. Serum α-SU and TRH stimulation tests showed great diagnostic sensitivity. Careful consideration is needed in diagnosing TSH PitNET. Achieving remission requires complete tumor resection. In case of nonremission, radiotherapy or medical therapy can improve the long-term remission rate.

- Thyroid
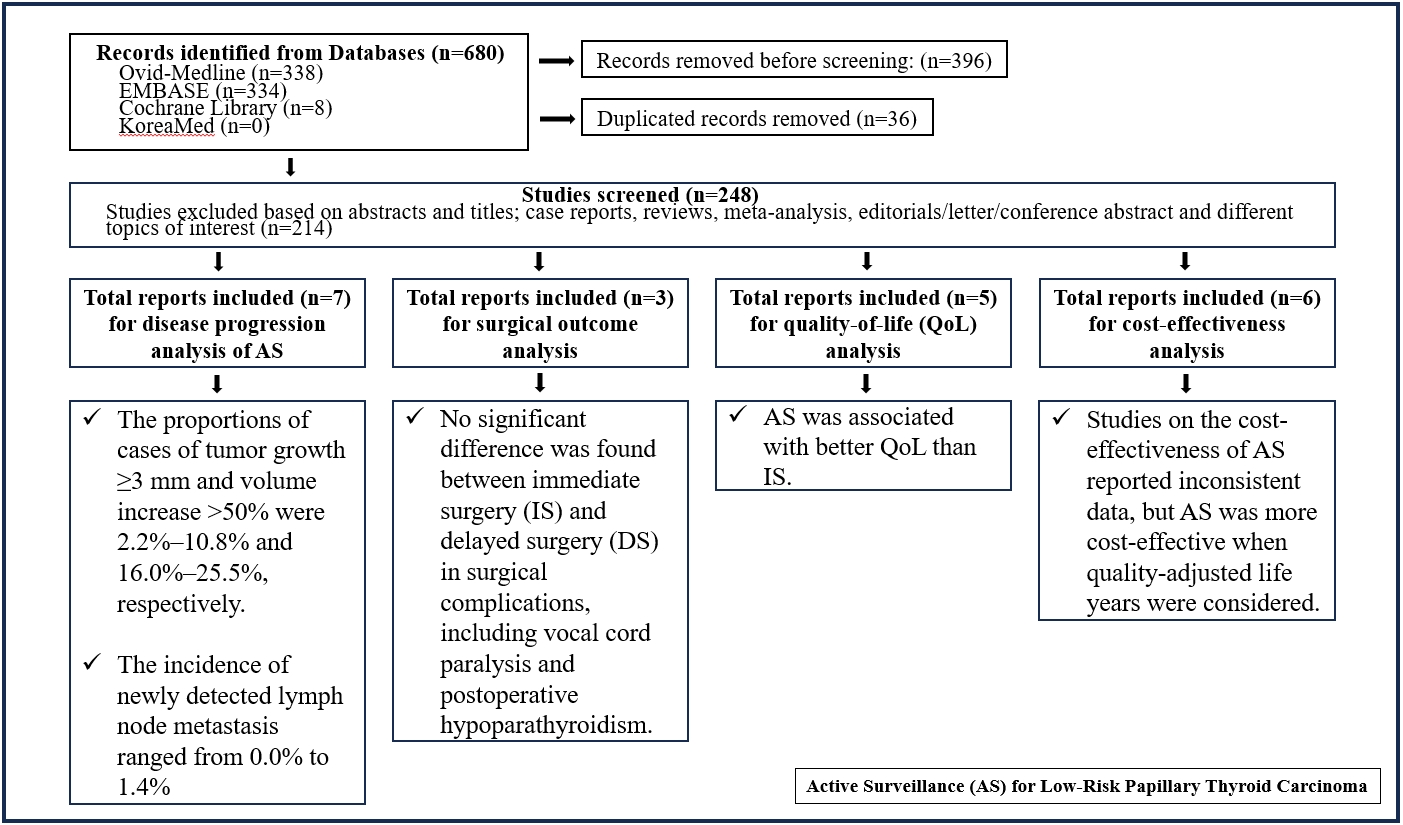
- Active Surveillance for Low-Risk Papillary Thyroid Carcinoma as an Acceptable Management Option with Additional Benefits: A Comprehensive Systematic Review
- Jee Hee Yoon, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2024;39(1):152-163. Published online January 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1794
- 1,146 View
- 41 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Active surveillance (AS) has been introduced as a management strategy for low-risk papillary thyroid carcinoma (PTC) due to its typically indolent nature. Despite this, the widespread adoption of AS has encountered several challenges. The aim of this systematic review was to evaluate the safety of AS related to disease progression and its benefits compared with immediate surgery (IS).
Methods
Studies related to AS in patients with low-risk PTC were searched through the Ovid MEDLINE, Embase, Cochrane Library, and KoreaMed databases. Studies on disease progression, surgical complication, quality of life (QoL), and cost-effectiveness were separately analyzed and narratively synthesized.
Results
In the evaluation of disease progression, the proportions of cases with tumor growth ≥3 mm and a volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Newly detected lymph node metastasis was identified in 0.0%–1.4% of patients. No significant difference was found between IS and delayed surgery in surgical complications, including vocal cord paralysis and postoperative hypoparathyroidism. AS was associated with better QoL than IS. Studies on the cost-effectiveness of AS reported inconsistent data, but AS was more cost-effective when quality-adjusted life years were considered.
Conclusion
AS is an acceptable management option for patients with low-risk PTC based on the low rate of disease progression and the absence of an increased mortality risk. AS has additional benefits, including improved QoL and greater QoL-based cost-effectiveness. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

- Thyroid
- The Diagnostic Role of Repeated Biopsy of Thyroid Nodules with Atypia of Undetermined Significance with Architectural Atypia on Core-Needle Biopsy
- Hye Hyeon Moon, Sae Rom Chung, Young Jun Choi, Tae-Yon Sung, Dong Eun Song, Tae Yong Kim, Jeong Hyun Lee, Jung Hwan Baek
- Endocrinol Metab. 2024;39(2):300-309. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1818
- 445 View
- 28 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to evaluate the utility of repeat biopsy of thyroid nodules classified as atypia of undetermined significance with architectural atypia (IIIB) on core-needle biopsy (CNB).
Methods
This retrospective study evaluated patients with thyroid nodules categorized as IIIB on CNB between 2013 and 2015. Demographic characteristics, subsequent biopsy results, and ultrasound (US) images were evaluated. The malignancy rates of nodules according to number of CNBs and the number of IIIB diagnoses was compared. Demographic and US features were evaluated to determine factors predictive of malignancy.
Results
Of 1,003 IIIB nodules on CNB, the final diagnosis was determined for 328 (32.7%) nodules, with 121 of them confirmed as malignant, resulting in a malignancy rate of 36.9% (95% confidence interval, 31.7% to 42.1%). Repeat CNB was performed in 248 nodules (24.7%), with 75 (30.2%), 131 (52.8%), 13 (5.2%), 26 (10.5%), one (0.4%), and two (0.8%) reclassified into categories II, IIIB, IIIA, IV, V, and VI, respectively. Malignancy rates were not significantly affected by the number of CNBs (P=0.291) or the number of IIIB diagnoses (P=0.473). None of the nodules confirmed as category II on repeat CNB was malignant. US features significantly associated with malignancy (P<0.003) included solid composition, irregular margins, microcalcifications, and high suspicion on the US risk stratification system.
Conclusion
Repeat biopsy of nodules diagnosed with IIIB on CNB did not increase the detection of malignancy but can potentially reduce unnecessary surgery. Repeat biopsy should be performed selectively, with US features guiding the choice between repeat biopsy and diagnostic surgery.

- Thyroid
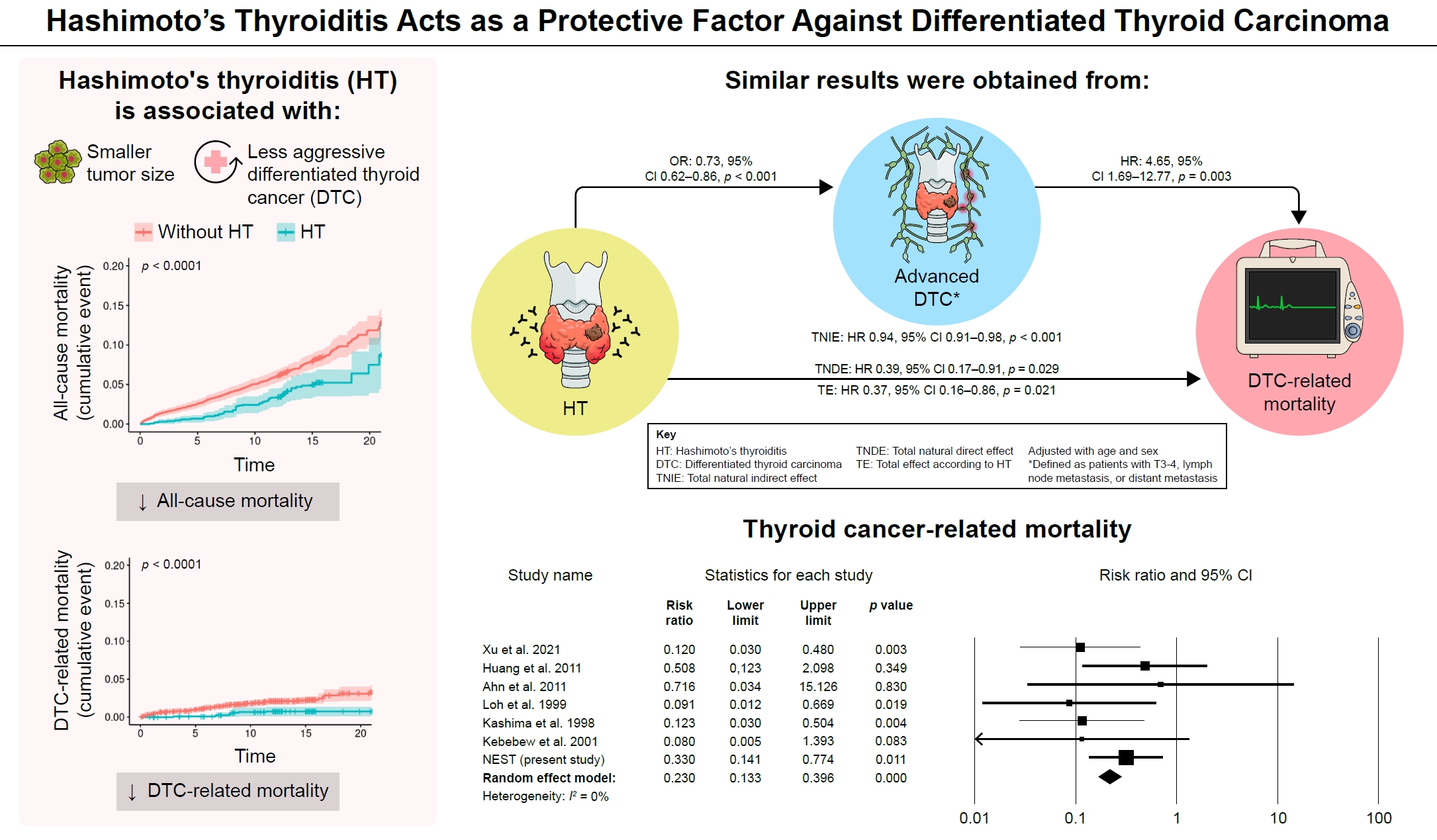
- Hashimoto Thyroiditis and Mortality in Patients with Differentiated Thyroid Cancer: The National Epidemiologic Survey of Thyroid Cancer in Korea and Meta-Analysis
- Injung Yang, Jae Myung Yu, Hye Soo Chung, Yoon Jung Kim, Yong Kyun Roh, Min Kyu Choi, Sung-ho Park, Young Joo Park, Shinje Moon
- Endocrinol Metab. 2024;39(1):140-151. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1748
- 998 View
- 50 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Many studies have shown that Hashimoto’s thyroiditis (HT) acts as a protective factor in differentiated thyroid cancer (DTC), but little is known about its effects on mortality. Therefore, this study was performed to reveal the prognosis of HT on mortality in patients with DTC.
Methods
This study included two types of research results: retrospective cohort study using the National Epidemiologic Survey of Thyroid cancer (NEST) in Korea and meta-analysis study with the NEST data and eight selected studies.
Results
Of the 4,398 patients with DTC in NEST, 341 patients (7.8%) died during the median follow-up period of 15 years (interquartile range, 12.3 to 15.6). Of these, 91 deaths (2.1%) were related to DTC. HT was associated with a smaller tumor size and less aggressive DTC. In Cox regression analysis after adjusting for age and sex, patients with HT showed a significantly lower risk of all-cause death (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.52 to 0.96) and DTC-related death (HR, 0.33; 95% CI, 0.14 to 0.77). The analysis with inverse probability of treatment weight data adjusted for age, sex, and year of thyroid cancer registration showed similar association. The meta-analysis showed that patients with HT showed a lower risk of all-cause mortality (risk ratio [RR], 0.24; 95% CI, 0.13 to 0.47) and thyroid cancer-related mortality (RR, 0.23; 95% CI, 0.13 to 0.40) in comparison with patients without HT.
Conclusion
This study showed that DTC co-presenting with HT is associated with a low risk of advanced DTC and presents a low risk for all-cause and DTC-related death.

Review Article
- Miscellaneous
- Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
- Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
- Endocrinol Metab. 2023;38(6):619-630. Published online November 21, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1814

- 2,451 View
- 110 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolism is a dynamic network of biochemical reactions that support systemic homeostasis amidst changing nutritional, environmental, and physical activity factors. The circulatory system facilitates metabolite exchange among organs, while the endocrine system finely tunes metabolism through hormone release. Endocrine disorders like obesity, diabetes, and Cushing’s syndrome disrupt this balance, contributing to systemic inflammation and global health burdens. They accompany metabolic changes on multiple levels from molecular interactions to individual organs to the whole body. Understanding how metabolic fluxes relate to endocrine disorders illuminates the underlying dysregulation. Cancer is increasingly considered a systemic disorder because it not only affects cells in localized tumors but also the whole body, especially in metastasis. In tumorigenesis, cancer-specific mutations and nutrient availability in the tumor microenvironment reprogram cellular metabolism to meet increased energy and biosynthesis needs. Cancer cachexia results in metabolic changes to other organs like muscle, adipose tissue, and liver. This review explores the interplay between the endocrine system and systems-level metabolism in health and disease. We highlight metabolic fluxes in conditions like obesity, diabetes, Cushing’s syndrome, and cancers. Recent advances in metabolomics, fluxomics, and systems biology promise new insights into dynamic metabolism, offering potential biomarkers, therapeutic targets, and personalized medicine.
-
Citations
Citations to this article as recorded by- Editorial: Tumor metabolism and programmed cell death
Dan-Lan Pu, Qi-Nan Wu
Frontiers in Endocrinology.2024;[Epub] CrossRef
- Editorial: Tumor metabolism and programmed cell death

Original Article
- Miscellaneous
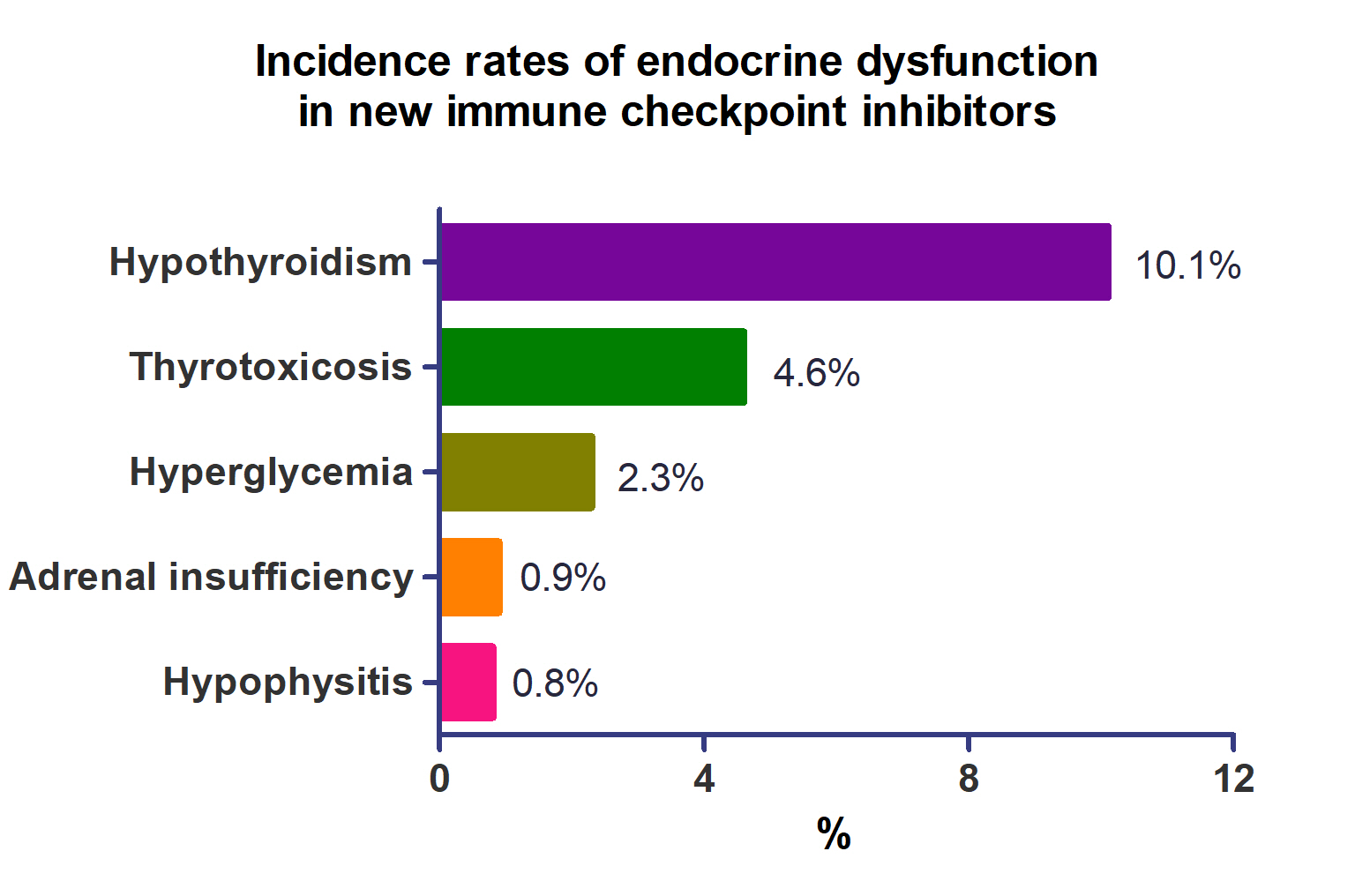
- Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
- Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
- Endocrinol Metab. 2023;38(6):750-759. Published online November 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1785
- 1,436 View
- 120 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the incidence of endocrine immune-related adverse events (irAEs) for recently developed immune checkpoint inhibitor (ICI) drugs.
Methods
We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through January 31, 2023. Among ICI drugs, nivolumab, pembrolizumab, and ipilimumab were excluded from the new ICI drugs because many papers on endocrine-related side effects have already been published.
Results
A total of 44,595 patients from 177 studies were included in this analysis. The incidence of hypothyroidism was 10.1% (95% confidence interval [CI], 8.9% to 11.4%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%). Hypothyroidism and thyrotoxicosis occurred most frequently with programmed cell death protein-1 (PD-1) inhibitors (13.7% and 7.5%, respectively). The rate of endocrine side effects for the combination of a programmed death-ligand 1 inhibitor (durvalumab) and cytotoxic T lymphocyte-associated antigen 4 inhibitor (tremelimumab) was higher than that of monotherapy. In a meta-analysis, the combination of tremelimumab and durvalumab had a 9- to 10-fold higher risk of pituitary and adrenal-related side effects than durvalumab alone.
Conclusion
Newly developed PD-1 inhibitors had a high incidence of thyroid-related irAEs, and combined treatment with durvalumab and tremelimumab increased the risk of pituitary- and adrenal-related irAEs. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.


 KES
KES

 First
First Prev
Prev



